All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Alternative biomarkers for assessing response to treatment in patients with LN
Measuring proteinuria is a commonly used approach for monitoring response to treatment and disease progression in patients with lupus nephritis (LN); however, proteinuria is not an ideal marker for kidney disease given the lack of correlation between proteinuria and the level of inflammation. Also, this approach is limited by an inability to differentiate between chronic damage and inflammation that may be treatable as well as different classes of kidney disease.
Kidney biopsy, whilst more informative, is not suitable for frequent sampling due to the invasive nature of the procedure, the number of contraindications that prohibit its use, and the timeframe of sample analysis. There remains a need for sensitive biomarkers to monitor inflammation and kidney damage in patients with LN.
During the American College of Rheumatology (ACR) annual meeting (ACR Convergence 2022), Fava presented data on urinary biomarkers that predict response to treatment in patients with LN that outperformed proteinuria.
Study design
For this study, 225 patients with systematic lupus erythematosus (SLE) who were undergoing a kidney biopsy due to proteinuria levels were enrolled, along with ten healthy donors. The study design is shown in Figure 1.
Figure 1. Study design*
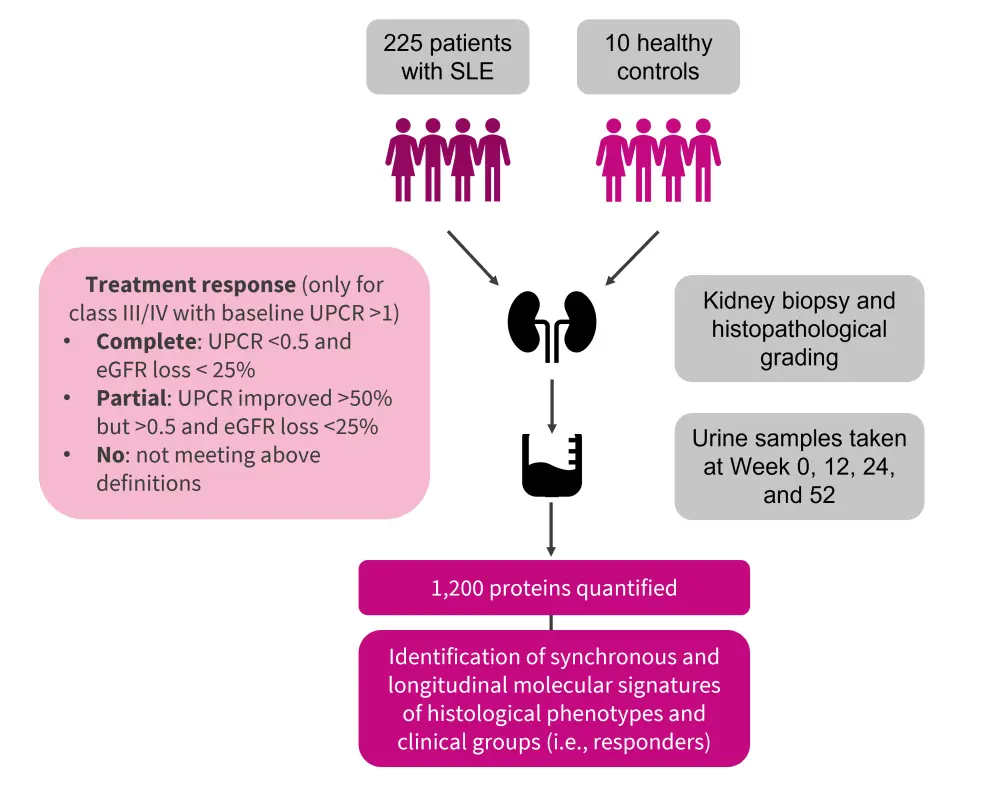
eGFR, estimated glomerular filtrate rate; SLE, systematic lupus erythematosus; UPCR, urine protein creatinine ratio.
*Adapted from Fava, et al.1
Results
Classification of the kidney biopsy samples are presented in Table 1, demonstrating that most patients had proliferative, pure membranous, or mixed class.
Table 1. Classification of kidney biopsy samples*
|
*Adapted from Fava, et al.1 |
|
|
Histopathological classification, %† |
|
|---|---|
|
Mesangial only (I–II) |
9 |
|
Pure membranous (V) |
25 |
|
Mixed (III or IV +/− V) |
24 |
|
Proliferative (III or IV) |
38 |
|
Advanced sclerosis (VI) |
4 |
Identifying biomarkers
The investigators also evaluated whether response to treatment could be determined from urine proteomics data at the time of biopsy, with no difference found between responders and non-responders.
However, assessing urine proteomics at 3 months showed a decline in several proteins in responders compared with non-responders. This protein signature was similar to a previously identified urine protein signature that correlated with the degree of histological activity in the kidney at the time of biopsy (Figure 2).
Figure 2. Urinary biomarkers signature proteins*
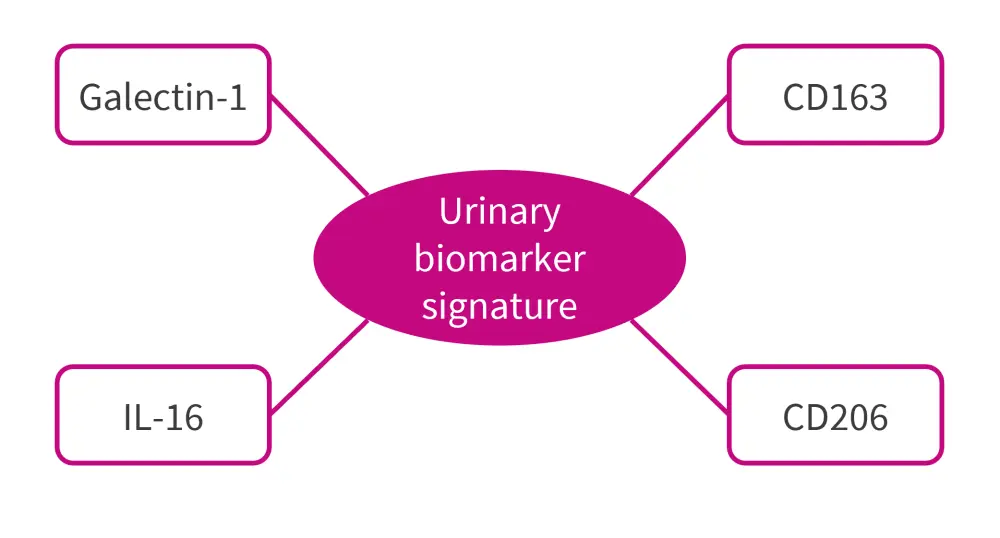
IL, interleukin.
*Adapted from Fava, et al.1
The protein signature persisted at 6 and 12 months. Pathway enrichment analysis demonstrated that an initial decrease in factors associated with the destruction of the extracellular matrix at 3 months was followed by a greater reduction in inflammatory markers at 6 and 12 months.
Assessing value of identified biomarkers
An assessment of the validity of these biomarkers found protein signatures at 3 months had a greater predictive power of 12-month treatment response than proteinuria. In particular, urinary CD206 significantly outperformed proteinuria as a biomarker for treatment response at 1 year, with an area under the curve of 91% (Table 2). The performance of these biomarkers was particularly striking in patients with proliferative LN.
CD206 and CD163 are both M2 macrophage markers that are shed during inflammation.
Table 2. AUC for urinary biomarkers*
|
AUC, area under the curve; UPCR, urine protein creatinine ratio. |
||
|
Biomarker |
AUC |
p value |
|---|---|---|
|
UPCR decrease |
0.78 |
0.0037 |
|
uCD163 decrease |
0.86 |
0.00026 |
|
uCD206 decrease |
0.91 |
2.9 ×10−5 |
Conclusion
This study found that urinary biomarkers were a better predictor of 12-month response than proteinuria in patients with LN, especially in cases of proliferative LN. Biomarkers of internal inflammation were shown to change in parallel to treatment response, allowing for earlier detection of treatment response or failure and enabling earlier intervention where treatment plans may need to be changed. While longitudinal studies are required to confirm the results of this study, and to assess the decline of kidney function at 3–5 years, these biomarkers could be suitable as surrogate endpoints for clinical trials.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

