All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Deucravacitinib in patients with SLE: Results from the phase II PAISLEY trial
The results of the phase II Paisley trial (NCT03252587) of deucravacitinib in patients with systemic lupus erythematosus (SLE) were presented at the American College of Rheumatology (ACR) annual meeting (ACR Convergence 2022) by Pike.1 This data has also been published in Arthritis and Rhemumatology.2
Deucravacitinib, an oral tyrosine kinase 2 (TYK2) inhibitor, received approval from the U.S. Food and Drug Administration (FDA) for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, as reported by the Psoriasis and Psoriatic Arthritis Hub.
Study design
This study included 363 patients with SLE who had moderate to severe disease, the study design is shown in Figure 1. Patients were randomized 1:1:1:1 between the four arms of the study. The primary endpoint was an SLE Responder Index (SRI) of 4 at Week 32.
Figure 1. Study design*
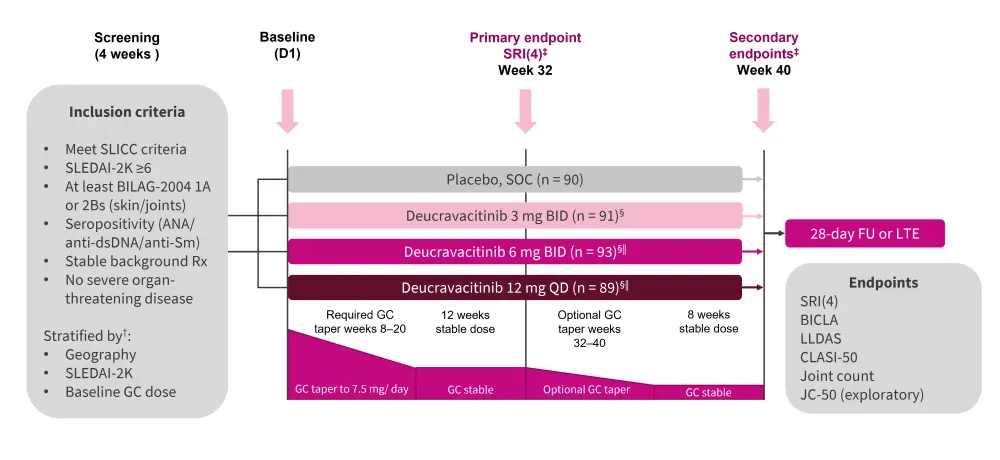
ANA, antinuclear antibody; anti-Sm, anti-smith; anti-dsDNA, anti-double stranded DNA; BID, twice daily; BILAG, British Isles Lupus Assessment Group; CLASI-50, Cutaneous Lupus Assessment Erythematosus Disease Area and Severity Index; FU, follow-up; GC, glucocorticoid; JC, joint count; JC-50, ≥50% improvement from baseline active joint counts; LLDAS, Lupus Low Disease Activity State; LTE, long-term extension; QD, once daily; SLE, systematic lupus erythematosus; SLEDAI-2k, SLE Disease Activity Index; SLICC, Systematic Lupus International Collaborating Clinics; SOC, standard of care; SRI, SLE Responder Index.
*Adapted from Pike.1
†Stratification factors included GC dose (≥10 mg/day or <10 mg/day), SLEDAI-2K score (≥10 or <10) and region (United States of America, Latin America, rest of World, and Japan [other stratification factors were not applied in Japan]).
‡Assessed by non-responder imputation adjusted for multiplicity.
§Type I error (alpha value) rate was allocated unequally between the 3 mg BID dose (alpha = 0.015), 6 mg BID dose (alpha = 0.025), and 12 mg QD dose (alpha = 0.01).
‖6 mg BID and 12 mg QD are the same daily dose with different pharmacokinetic characteristics.
Results
Patient characteristics and disposition
Baseline patient characteristics were similar across the four groups (Table 1). The mean SLEDAI-2K score for the whole cohort was 10.8 and patients on average had ten swollen or ‘active’ joints. Around 50% of patients were on immunosuppressants across all groups assessed; however, this was slightly higher in the deucravacitinib 3 mg twice daily group at 58.2%. There was a higher percentage of patients being treated with antimalarials, immunosuppressants, and glucocorticoids concurrently in the deucravacitinib 3 mg twice daily group (41.8%) than in the placebo group (28.9%) and other treatment groups (6 mg twice daily, 28.0%; 12 mg once daily, 30.3%).
Table 1. Baseline patient characteristics*
|
BID, twice daily; BILAG-2004, British Isles Lupus Assessment Group 2004 Index; BMI, body mass Index; CLASI-A, Cutaneous Lupus Erythematosus Disease Area and Severity Index Activity; GC, glucocorticoid; PGA, Physician’s Global Assessment; QD, once daily; SD, standard deviation; SLEDAI-2K, SLE Disease Activity Index 2000. |
||||
|
Characteristic |
Placebo (n = 90) |
Deucravacinitib |
Deucravacinitib |
Deucravacinitib |
|---|---|---|---|---|
|
Mean age (SD), |
40.1 (13.1) |
40.2 (11.9) |
40.9 (12.5) |
40.1 (10.6) |
|
Mean BMI (SD), |
27.5 (6.7) |
26.5 (6.7) |
26.1 (6.9) |
27.1 (6.9) |
|
Female, % |
88.9 |
93.4 |
94.6 |
91.0 |
|
White, % |
66.7 |
68.1 |
59.1 |
64.0 |
|
Treatment, % |
|
|
|
|
|
GC |
82.2 |
81.3 |
78.5 |
79.8 |
|
≥10 mg/day |
52.2 |
49.5 |
49.5 |
48.3 |
|
Antimalarial |
83.3 |
89.0 |
90.3 |
84.3 |
|
Immunosuppressant |
51.1 |
58.2 |
46.2 |
51.7 |
|
Antimalarial, |
28.9 |
41.8 |
28.0 |
30.3 |
|
SLEDAI-2K (SD), mean |
10.8 (3.1) |
11.1 (3.2) |
10.8 (3.2) |
10.7 (3.0) |
|
Overall BILAG-2004, % |
56.7 |
56.0 |
47.3 |
57.3 |
|
Mean PAG (SD), n |
1.82 (0.4) |
1.80 (0.3) |
1.84 (0.4) |
1.86 (0.4) |
|
Mean CLASI-A score (SD), n |
8.0 (5.1) |
8.6 (7.6) |
8.2 (6.5) |
8.4 (5.8) |
|
Mean CLASI-A score in |
14.9 (4.2) |
18.2 (9.7) |
16.0 (7.5) |
15.6 (3.6) |
|
Mean active joint count§ (SD), n |
9.2 (6.0) |
8.6 (4.8) |
8.8 (6.1) |
9.4 (5.7) |
Between 69.7 and 81.7% of patients finished their course of treatment in their respective groups. Adverse events (AEs) were the most common reason cited for discontinuation in the treatment group (6.5–13.5% in treatment arms vs 3.3% in placebo) compared with lack of efficacy for the placebo group (2.2–4.5% in treatment arms vs 7.8% in placebo group).
Efficacy
Primary endpoint
Response rates had the highest significant increase in patients receiving 3 mg of deucravacinitib twice a day at Week 32 compared with the placebo (p = 0.0006; Figure 2). The median time to first onset of SRI 4 response was 116 days (95% confidence interval [CI], 112–144) in the placebo group compared with 85 (range, 85–113 days), 92 (range, 85–138 days), and 111 (range, 85−115 days) days in the deucravacinitib 3 mg twice daily, 6 mg twice daily, and 12 mg once daily groups, respectively.
Figure 2. SRI 4 response rate at Week 32*†
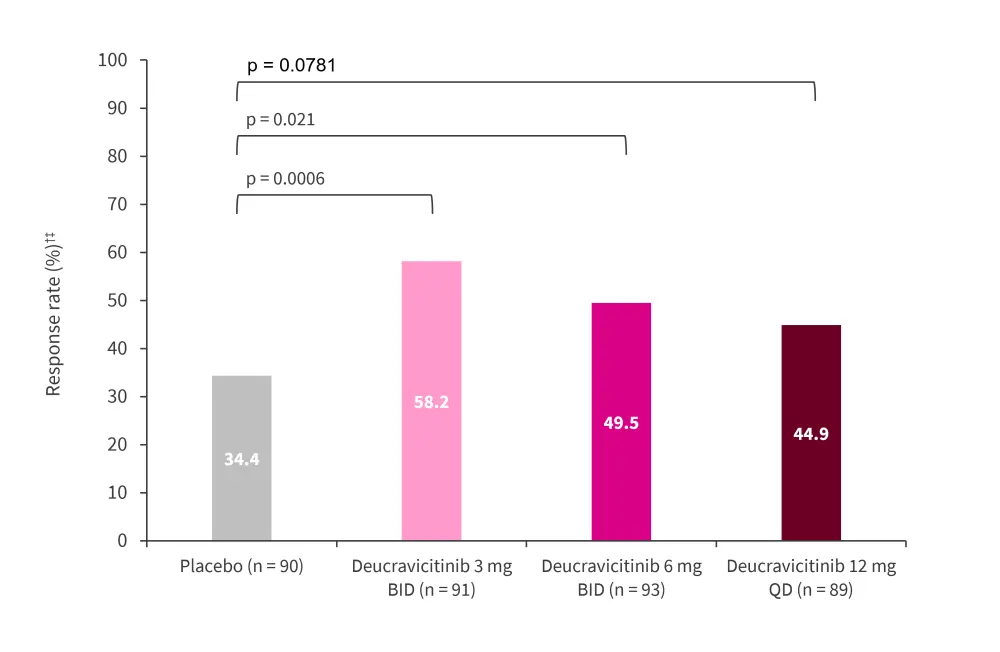
BICLA, British Isles Lupus Assessment Group-based Composite Lupus Assessed; BID, twice daily; QD, once daily; SRI, Systemic Lupus Erythematosus Responder Index.
*Adapted from Pike.1
†Assessed by non-responder imputation; all randomized patients assessed with instances of missing data, prohibited medication use, or early discontinuation analyzed as a nonresponse.
‡p value was significant vs placebo in multiplicity controlled prespecified analysis.
Secondary endpoints
The greatest significant increase in response rates for SRI 4 and British Isles Lupus Assessment Group-based composite Lupus assessed (BICLA) at Week 48 was achieved in the 3 mg twice daily group (Figure 3A and B).
Patients treated with deucravacitinib, regardless of dosing regimen, had an increased response rate compared with placebo in Lupus low disease activity state (LLDAS) and Cutaneous Lupus Erythematosus Disease Area and Severity Index Activity (CLASI)-50 (patients with a CLASI score ≥10 who have ≥50% decrease from baseline) at Week 48 (Figure 3C and D).
A further secondary endpoint was the mean change in active joints (which included both swollen and tender joints) from baseline; this is shown in Figure 3E. Although a placebo effect was noted, there was a statistically significant decrease in the number of active, swollen, or tender joints in the 3 mg twice daily deucravacitinib group compared with the placebo group.
Figure 3. Secondary endpoints measured at Week 48: A SRI 4†, B BICLA†, C LLDAS†, D CLASI-50†, and E active, swollen, and tender joint counts*
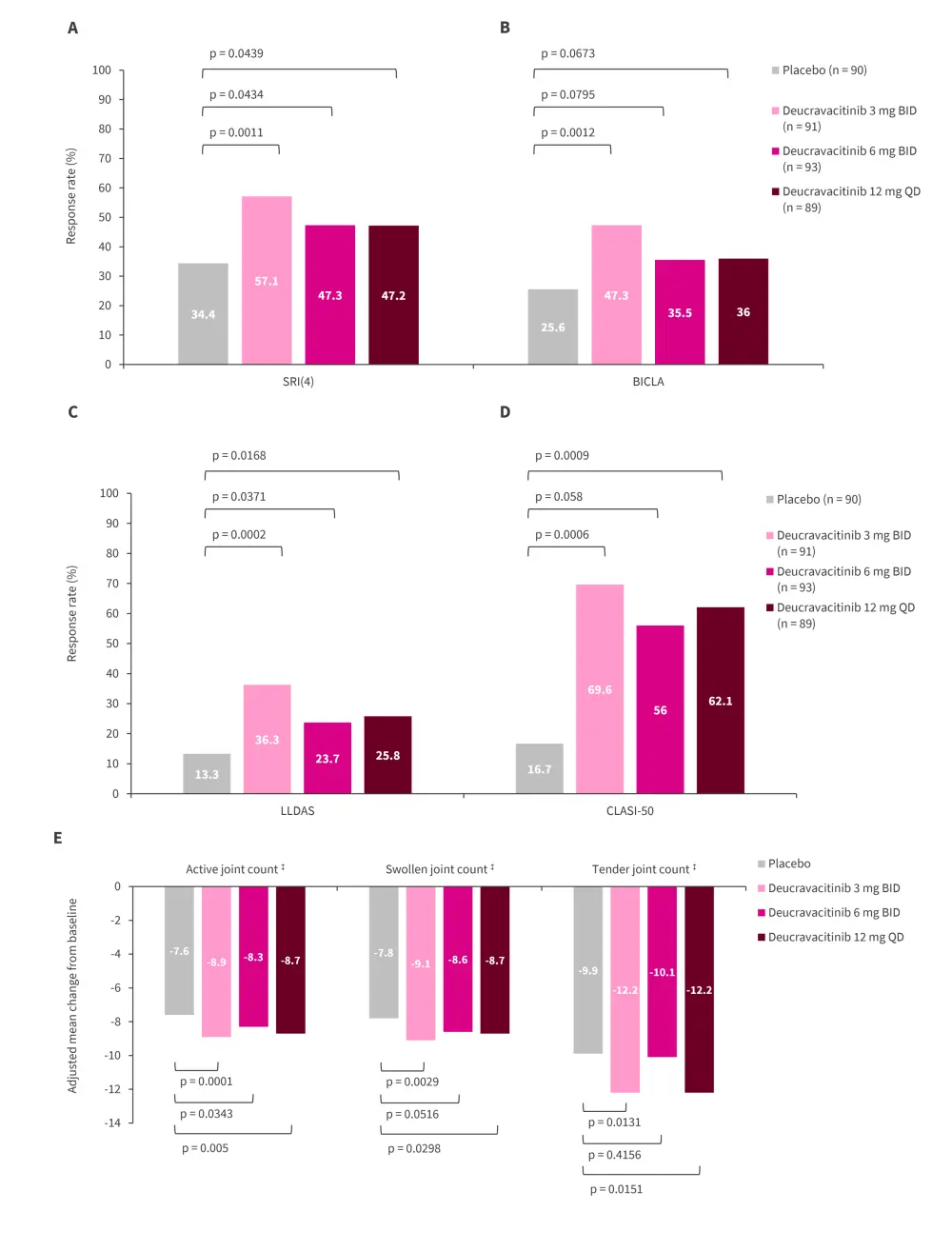
BICLA, British Isles Lupus Assessment Group-based Composite Lupus Assessed; BID, twice daily; CI, confidence interval; CLASI-50, Cutaneous Lupus Erythematosus Disease Area and Severity Index Activity; LLDAS, lupus low disease activity state; QD, once daily; SRI, Systemic Lupus Erythematosus Responder Index.
*Adapted from Pike.1
†Assessed by non-responder imputation. All randomized patients assessed; missing data, prohibited medication use, or early discontinuation analyzed as a nonresponse. Number of patients in each group were: placebo n = 90, deucravacitinib 3 mg BID n = 91, deucravacitinib 6 mg BID n = 93, and deucravacitinib 12 mg QD n = 89.
‡The adjusted mean change in each joint count was calculated using a mixed model repeated measures model. For analysis visits where the non-responder imputation criteria were met, the observed values were set to missing and the overall cohort response modeled. Active joint count included swollen and tender joints.
Safety
Serious AEs remained similar in the treatment groups compared with placebo, with the main AEs recorded being infections or infestations (Figure 4). Skin related AEs, such as acne and rashes, were elevated in the 6 mg twice daily and 12 mg once daily treatment groups at 34.4% and 33.7%, respectively. No cardiac or thromboembolic events were recorded (Figure 5).
Figure 4. Safety results from Week 0 to 48*
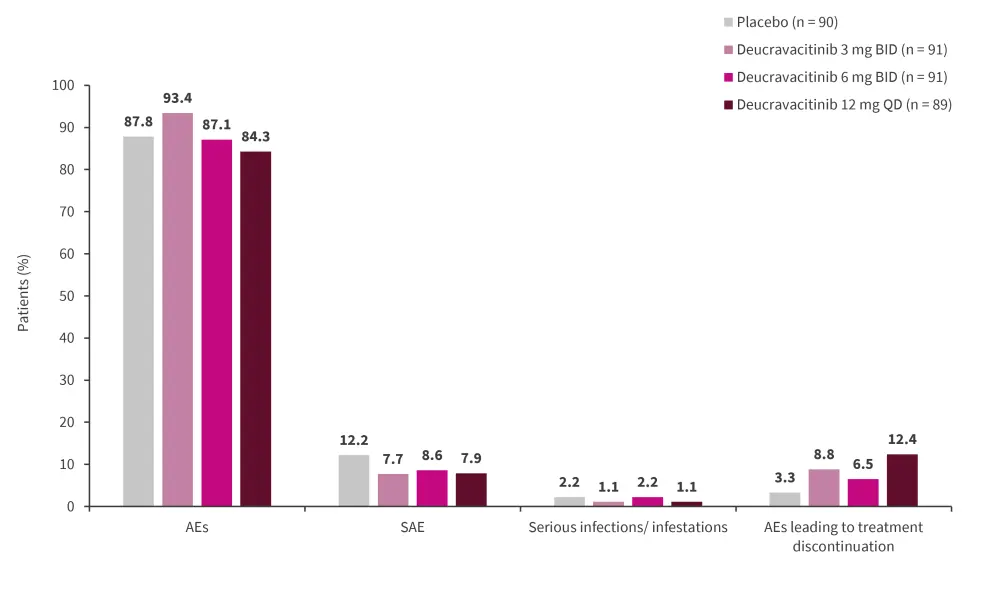
*Adapted from Pike.1
AE, adverse event; BID, twice daily; SAE, severe AE; QD, once daily.
Figure 5. AEs of interest*
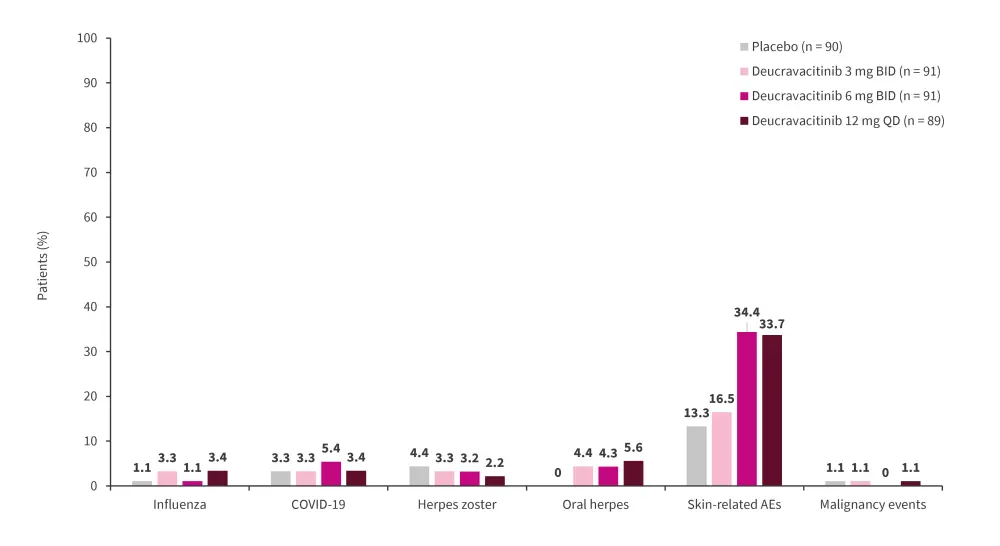
*Adapted from Pike.1
AE, adverse event; BID, twice daily; SAE, severe AE; QD, once daily.
Laboratory parameters
Treatment with deucravacitinib had no effect on hematological parameters such as hemoglobin, neutrophil, lymphocyte, and platelet levels. Renal parameters, such as alanine aminotransferase and creatinine levels, were also not affected by treatment across the different dosing groups.
Conclusion
This trial reached its primary endpoint (SRI of 4) compared with placebo using deucravacitinib; all secondary endpoints were achieved or improved at Week 48. Higher doses of deucravacitinib, beyond 3 mg twice daily, did not show any additional benefit. Safety results were consistent with previous psoriasis trials, most AEs were mild, and there was no evidence of abnormalities in laboratory parameters associated with Janus kinase inhibition. The investigators noted that deucravacitinib warrants further assessment in a phase III study.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

