All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
EULAR recommendations for infection prevention and vaccination administration in adult patients with AIIRD
Patients with autoimmune inflammatory diseases (AIIRD) are frequently treated with immunosuppressive agents, which can leave them vulnerable to opportunistic and chronic infections.1 Screening for infections and prophylactic measures are required to help protect these patients.1
In this article, we summarize the 2022 European Alliance of Associations for Rheumatology (EULAR) recommendations published by Fragoulis et al.1 for screening and prophylaxis of infections in adult patients with AIIRD. This article will also provide an update on the vaccination recommendations in this patient population.2
EULAR recommendations for screening and prophylaxis of chronic and opportunistic infections in patients with AIIRD1
The 2022 EULAR recommendations were developed by an international task force of 22 members from 15 different countries. Figure 1 highlights the overarching principles employed as rationale behind the recommendations, which are listed in Figure 2. All four of the overarching principles had a mean level of agreement (ranging from 0 [no agreement] to 10 [full agreement]) ≥9.5 among members of the task force.
Figure 1. 2022 EULAR recommendations: Overarching principles*
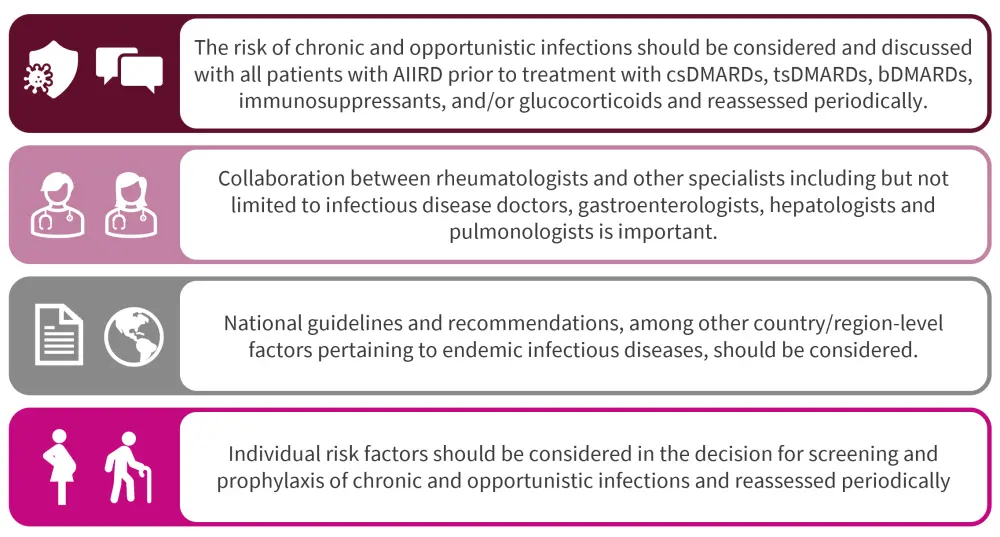
AIIRD, autoimmune inflammatory rheumatic diseases; bDMARD, biological disease-modifying antirheumatic drug; csDMARD, conventional synthetic disease-modifying antirheumatic drug; DMARD, disease modifying antirheumatic drug; EULAR, European Alliance of Associations for Rheumatology; tsDMARD, targeted synthetic disease-modifying antirheumatic drug.
*Adapted from Fragoulis, et al.1
All EULAR recommendations had a high level of agreement within the task force with a range of 8.9−9.5 (Figure 2).
Figure 2. 2022 EULAR recommendations for prevention of opportunistic and chronic infections in adults with AIIRD*
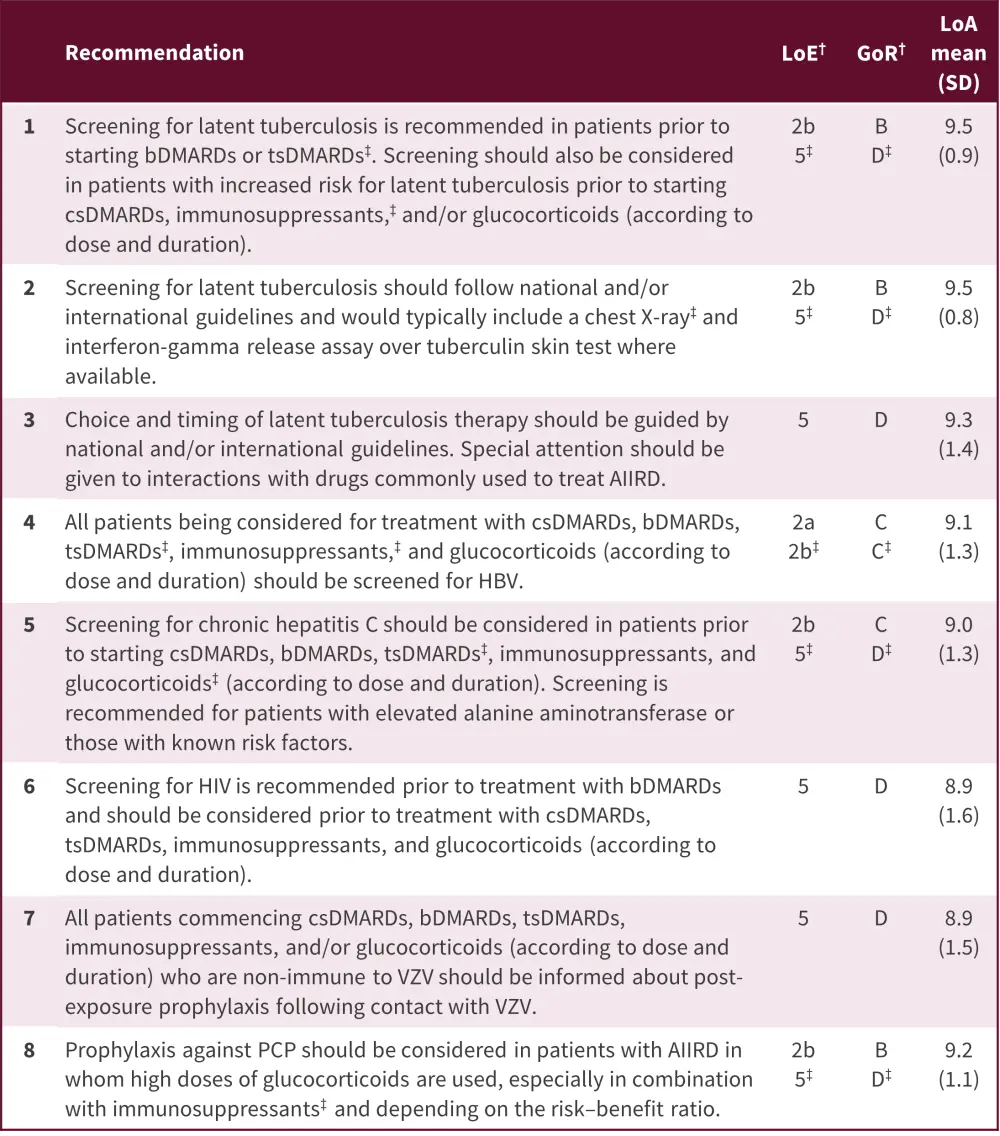
AIIRD, autoimmune inflammatory rheumatic diseases; bDMARDs, biological disease-modifying antirheumatic drugs; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; EULAR, European Alliance of Associations for Rheumatology; GoR, grade of recommendation; HBV, hepatitis B virus; LoA, level of agreement; LoE, level of evidence; PCP, pneumocystis pneumonia; tsDMARDs, targeted synthetic disease-modifying antirheumatic drugs; VZV, varicella zoster virus.
*Adapted from Fragoulis, et al.1
†Based on the Oxford Evidence Based Medicine categorization, as per EULAR guidance.
‡Denotes separate LoE and GoR, where this is different from the rest of the statement.
All patients considered for treatment with csDMARDs, bDMARDs, tsDMARDs, immunosuppressants, and glucocorticoids (according to dose and duration) should be screened for HBV
Recommendation 4 in the 2022 EULAR list relates to screening for hepatitis B virus (HBV). Due to the complexity of this process, a figure is included (Figure 3) along with this recommendation.
Figure 3. Recommended screening schedule for HBV*
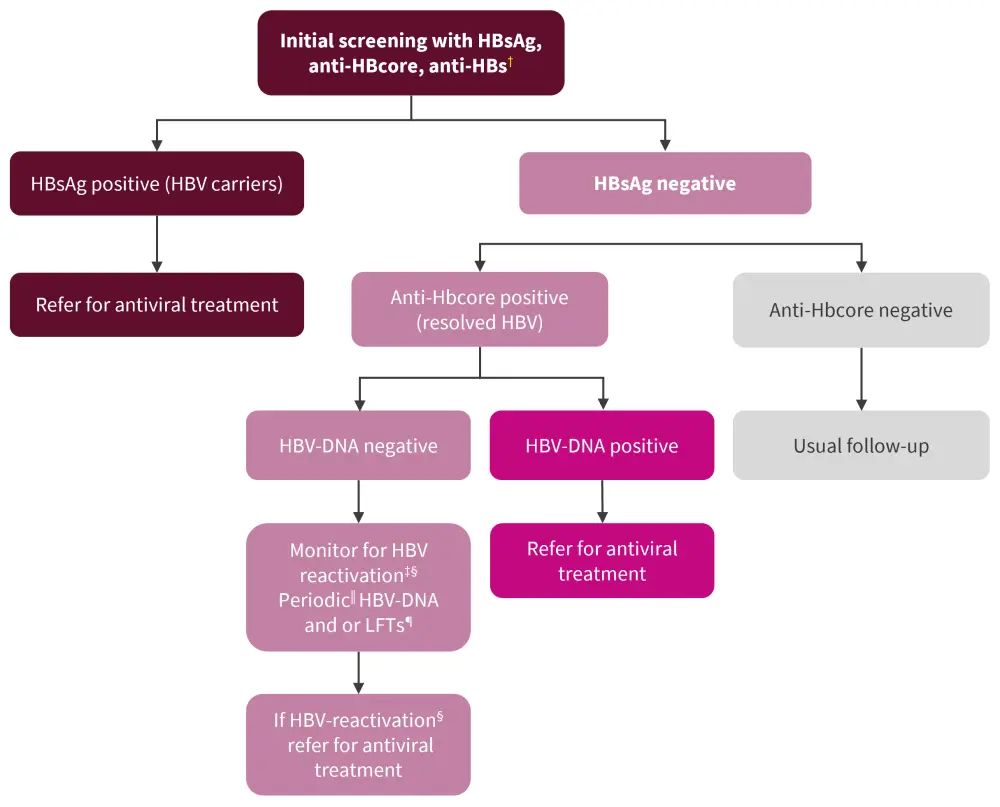
anti-HBs; hepatitis B surface antibody; DNA, deoxyribonucleic acid; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; LFT, liver function test.
*Adapted from Fragoulis, et al.1
†Positive anti-HBs without positive HBsAg or anti-HBcore is consistent with prior vaccination. If all three (HBsAg, anti-HBcore, anti-HBs) are negative, means no previous exposure to HBV.
‡Consider referral for antiviral prophylaxis for those commencing rituximab, having also low titers of anti-HBs. Risk is assessed on an individual basis.
§HBV-reactivation: rise or appearance of HBV-DNA, or conversion from HBsAg-negative to HBsAg-positive.
‖Periodic: there are no data to specify the exact time at which re-screening for HBV-reactivation should be performed. However, every 3–6 months is the standard for many national guidelines. Risk factors and cost should also be considered.
¶Referral to hepatologists is also recommended.
EULAR 2019 update on vaccination in adult patients with AIIRD2
The 2019 update on vaccination recommendations published by Furer, et al.2 was created after conducting four systematic literature reviews and based on the opinions of the task force involved. For this update, six overarching principles were identified (Figure 4) and used to formulate nine recommendations (Figure 5).
All overarching principles had high level of agreement (>93%) except for the principle related to live-attenuated vaccines. While the 2011 EULAR recommendations stated that live vaccines should be avoided whenever possible, the 2019 update has been changed to allow more flexibility as a result of new evidence and experience with the use of live vaccines in this patient population. The recommended time to vaccinate a patient with AIIRD is 4 weeks prior to the start of treatment. The measles, mumps, and rubella booster and attenuated herpes zoster vaccines are highlighted as possible exceptions to avoiding vaccination when patients are treated with immunosuppressive agents.
Figure 4. EULAR 2019 vaccination recommendations: Overarching principles*

AIIRD, autoimmune inflammatory rheumatic diseases; DMARD, disease-modifying antirheumatic drug; EULAR, European Alliance of Associations for Rheumatology.
*Adapted from Furer, et al.2
Figure 5. EULAR 2019 vaccination recommendations*
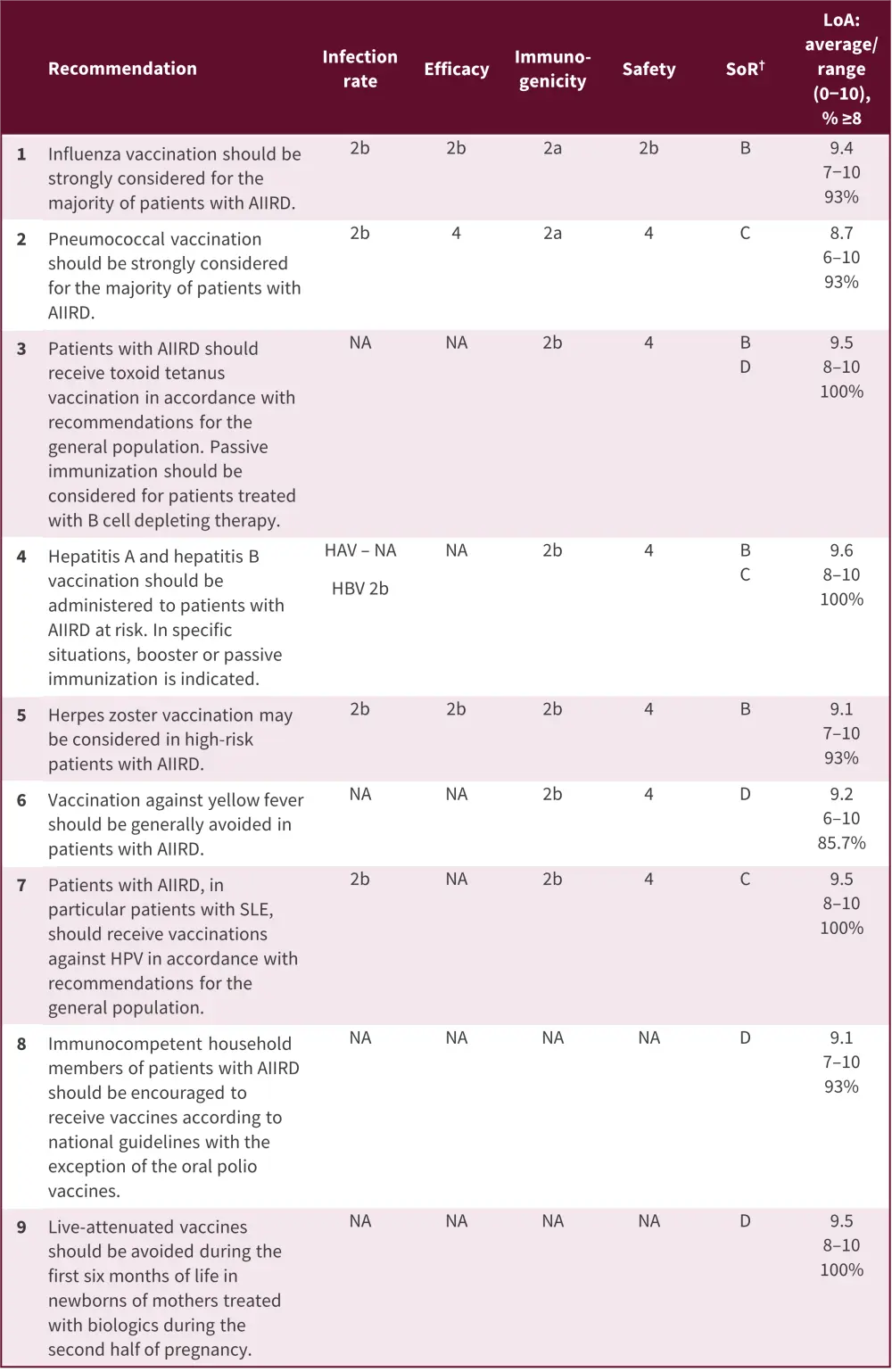
AIIRD, autoimmune inflammatory rheumatic diseases; EULAR, European Alliance of Associations for Rheumatology; HAV, hepatitis A virus; HBV, hepatitis B virus; HPV, human papilloma virus; LoA, level of agreement; NA, non-available; SLE, systemic lupus erythematosus; SoR, strength of recommendation.
*Adapted from Furer, et al.2
†Determined following the standards of the Oxford Centre for Evidence Based Medicine. The strength of recommendation was primarily based on the efficacy data. If no efficacy data were available, immunogenicity was used as a major outcome. When immunogenicity outcomes did not directly correlate with protection, the strength of recommendation was downgraded. Few recommendations were primarily based on safety (especially live-attenuated vaccines) and here, the safety outcomes determined the strength of the recommendation.
Conclusion
The 2022 recommendations on infection screening and prophylaxis are the first to be produced by EULAR to respond to an overarching need for guidance on prevention of infection in adult patients with AIIRD. These recommendations aimed to overcome differences in clinical practice and the limited availability of reference literature, which may act as barriers to optimal management of these patients.
Uptake of vaccinations is generally very low in patients with AIIRD; therefore, it is important to increase education and raise awareness among healthcare professionals and patients regarding evidence-based use and appropriate administration timing in this patient population.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

