All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Iberdomide in systemic lupus erythematosus: Results from a phase II study
Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune inflammatory disorder associated with the disruption of multiple innate and adaptive immune pathways.1
Iberdomide is a high affinity cereblon E3 ligase modulator that promotes proteasomal degradation of Ikaros (IKZF1) and Aiolos (IKZF3). Overexpression and genomic alteration of IKF1 and IKZF3 are strongly associated with the development of SLE.1
Here, we summarize the key findings from a phase II study (NCT03161483) of iberdomide in patients with active, moderate-to-severe SLE recently published by Merrill, et al.1 in the New England Journal of Medicine in 2022.
Study design
The trial was conducted at 117 sites in the United States, Canada, Europe, South America, Mexico, and Russia from July 6, 2017, to January 21, 2020, in patients with SLE.
Patients were included if they were aged ≥18 years with ≥6 points on the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI)-2K, a score of ≥4 on the clinical SLEDAI-2K (SLEDAI-2K without laboratory results), antinuclear antibody titers of at least 1:40, and seropositivity for anti-double-stranded DNA antibodies or anti-Smith antibodies.
Patients were treated once a day for 24 weeks with placebo (n = 83) or iberdomide at 0.45 mg (n = 82), 0.30 mg (n = 82), or 0.15 mg (n = 42).
Endpoints
- Primary endpoint was a response on the SLE Responder Index (SRI-4) at Week 24
- Secondary endpoints included the following:
- A decrease of ≥4 points from baseline in SLEDAI-2K score
- No new A scores or >1 B score on British Isles Lupus Assessment Group (BILAG)-2004
- No significant decrease in Physician Global Assessment (PGA) score, <0.3 change from baseline
- Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI)-50 in the subgroup of patients with CLASI score ≥10 at baseline
- Mean change from baseline in number of swollen joints or tender joints in the subgroup of patients with ≥2 affected joints at baseline
- Adjusted mean change from baseline in Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue score
- Exploratory endpoints included SRI-4 response in patients with a baseline SLEDAI-2K score of ≥10 and those with high expression of the Aiolos or type I interferon gene signatures
- Safety
Results
Baseline characteristics
A total of 288 patients were treated with placebo (n = 83) or iberdomide at 0.45 mg (n = 81), 0.30 mg (n = 82) or 0.15 mg (n = 42), and 247 (86%) completed the 24-week placebo-controlled period. A higher percentage of the patients in the iberdomide 0.30 mg group discontinued treatment due to adverse events (AEs) or withdrew from the trial compared with the other groups. Baseline characteristics were balanced among the treatment groups (Table 1).
Table 1. Baseline and clinical characteristics*
|
BILAG-2004, British Isles Lupus Assessment Group 2004; CLASI-A, Cutaneous Lupus Erythematosus Disease Area and Severity Index-Activity; dsDNA, double-stranded DNA; PGA, Physician Global Assessment; SLE, systemic lupus erythematosus; SLEDAI-2K; systemic Lupus Erythematosus Disease Activity Index-2000. |
|||||
|
Characteristic, % |
Ibe |
Ibe |
Ibe |
Placebo |
Total |
|---|---|---|---|---|---|
|
Mean age, years |
46.4 |
44.7 |
43.8 |
43.4 |
44.7 |
|
Female |
98 |
94 |
98 |
98 |
97 |
|
Race† |
|
|
|
|
|
|
Black |
6 |
7 |
7 |
8 |
7 |
|
White |
74 |
72 |
69 |
72 |
72 |
|
Other |
20 |
21 |
24 |
19 |
20 |
|
Hispanic or Latino ethnic group |
41 |
56 |
50 |
49 |
49 |
|
Geographic region |
|
|
|
|
|
|
US or Canada |
22 |
24 |
21 |
19 |
22 |
|
Europe |
38 |
22 |
26 |
33 |
30 |
|
Mexico or South America |
36 |
48 |
48 |
42 |
43 |
|
Russia |
4 |
6 |
5 |
6 |
5 |
|
Median time from initial diagnosis of SLE to randomization (range), years |
9.0 |
7.3 |
7.3 |
5.7 |
7.2 |
|
Antinuclear antibody level ≥1:80 |
98 |
100 |
100 |
100 |
99 |
|
Mean SLEDAI-2K global score‡ |
9.5 |
9.6 |
9.5 |
9.8 |
9.6 |
|
BILAG-2004, 1 A score or >1 B score§ |
73 |
73 |
83 |
78 |
76 |
|
Mean PGA score¶ |
1.7 |
1.7 |
1.7 |
1.7 |
1.7 |
|
Mean CLASI-A activity‖ |
7.2 |
7.1 |
7.2 |
6.3 |
6.9 |
|
Cutaneous lupus subtype |
|
|
|
|
|
|
Acute/subacute/chronic |
47/15/36 |
52/11/28 |
71/21/33 |
60/20/22 |
56/16/29 |
|
Number of affected joints |
|
|
|
|
|
|
Swollen/tender |
5.5/8.2 |
7.2/9.8 |
7.2/8.6 |
6.4/8.7 |
6.5/8.9 |
|
High gene signature |
|
|
|
|
|
|
Aiolos/type 1 |
44/70/79 |
39/60/65 |
33/60/67 |
33/58/68 |
38/62/70 |
|
Elevated anti-dsDNA antibody level |
36 |
28 |
31 |
33 |
32 |
|
Baseline treatment for SLE |
|
|
|
|
|
|
Any dose of oral |
72 |
78 |
74 |
77 |
75 |
|
Oral glucocorticoid |
40 |
37 |
40 |
37 |
38 |
|
Antimalarial agent |
62 |
77 |
67 |
80 |
72 |
|
Immunosuppressant |
46 |
44 |
52 |
41 |
45 |
Primary and secondary endpoints
At Week 24, a higher percentage of patients in the iberdomide 0.45 mg treatment group achieved an SRI-4 response than in the placebo group. The primary endpoint was not met with the other doses of iberdomide (0.30 mg and 0.15 mg) (Figure 1).
Figure 1. SLE Responder Index at Week 24 in the intention-to-treat population*
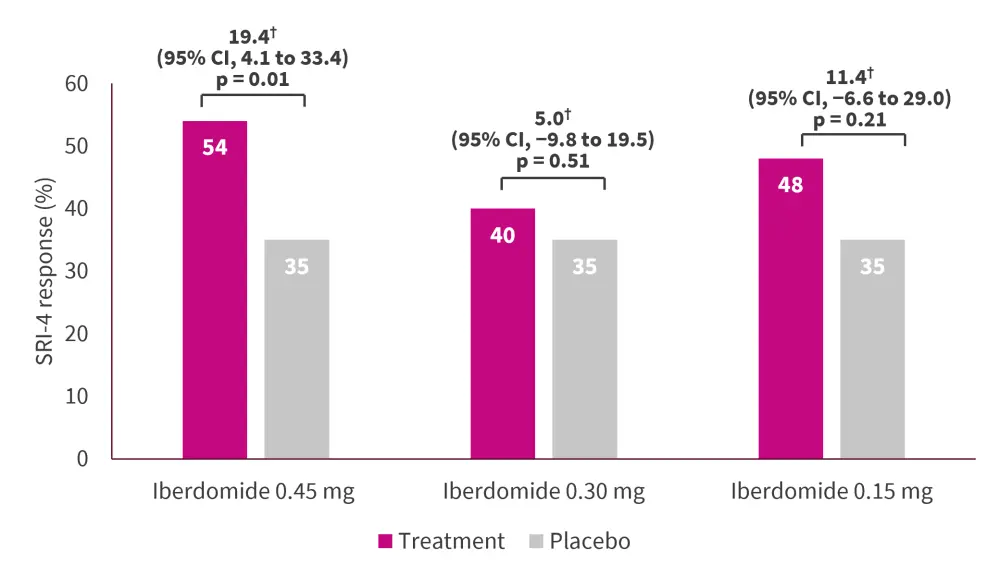
CI, confidence interval; SRI-4, Subcutaneous Lupus Erythematosus Responder Index. *Adapted from Merrill, et al.1
†Difference between treatment group and placebo.
Secondary endpoints in the iberdomide groups versus placebo are summarized in Table 2.
Table 2. Secondary efficacy endpoints at Week 24 in the intention-to-treat population*
|
BILAG-2004, British Isles Lupus Assessment Group 2004 Index; CLASI-A, Cutaneous Lupus Erythematosus Disease Area and Severity Index-Activity; FACIT-Fatigue, Functional Assessment of Chronic Illness Therapy-Fatigue; PGA, Physician Global Assessment; SLE, systemic lupus erythematosus; SLEDAI-2K; Systemic Lupus Erythematosus Disease Activity Index-2000. |
|||
|
Endpoint, difference in % points |
Iberdomide 0.45 mg vs placebo |
Iberdomide 0.30 mg vs placebo |
Iberdomide 0.15 mg vs placebo |
|---|---|---|---|
|
Decrease of ≥4 points from baseline in SLEDAI-2K score |
19.3 |
6.5 |
10.3 |
|
No new A scores or >1 B score on BILAG-2004 |
8.0 |
−5.3 |
12.4 |
|
No significant decrease in PGA score, <0.3 change from baseline |
6.8 |
−4.3 |
12.1 |
|
CLASI-50 in a subgroup of patients with CLASI score ≥10 at baseline |
14.2 |
5.3 |
24.0 |
|
Mean change from baseline in no. of swollen joints in a subgroup of patients with ≥2 swollen or tender joints at baseline† |
0.1 |
0.7 |
0.7 |
|
Mean change from baseline in no. of tender joints in a subgroup of patients with ≥2 swollen or tender joints at baseline† |
0.3 |
1.3 |
1.1 |
|
Adjusted mean change from baseline in FACIT-Fatigue score‡ |
1.4 |
−0.6 |
−1.1 |
|
Glucocorticoid dose reduced by week 16 to <10 mg/day from a dose ≥10 mg/day at baseline |
— |
−3.2 |
— |
At Week 24, a higher percentage of patients in the iberdomide 0.45 mg treatment group achieved a secondary endpoint compared with the placebo group.
- A decrease in SLEDAI-2K score of ≥4 points from baseline was observed in 56% of patients who received 0.45 mg compared with 36% of patients who received placebo.
- CLASI-50 was achieved in 68% of patients in the 0.45 mg group compared with 50% in the placebo group.
- The adjusted mean change from baseline in FACIT-Fatigue score was 5.2 in the 0.45 mg group compared with 3.8 in the placebo group.
No meaningful differences were observed in the BILAG-2004 and PGA scores at Week 24.
Exploratory endpoints
According to biomarkers at baseline, an SRI-4 response was observed in the iberdomide 0.45 mg, 0.30 mg, 0.15 mg, and placebo groups at the frequencies listed below.
- High Aiolos gene signature: 64%, 28%, 36% and 33%, respectively
- High type I interferon gene signature: 60%, 43%, 60%, and 33%, respectively
- High Ikaros expression: 55%, 40%, 43%, and 38%, respectively
Safety
Overall, 78%, 78%, 74%, and 65% in the 0.45 mg, 0.30 mg, 0.15 mg, and placebo groups, respectively, reported any AE (Table 3). Most events were mild to moderate severity. The most frequent AEs in the iberdomide groups were urinary tract infections, upper respiratory tract infections, neutropenia, and influenza. Serious AEs occurred in 6% of patients who received iberdomide and 8% of patients who received placebo (SLE flares). No deaths were reported in the iberdomide groups and one death was reported in the placebo group due to pulmonary embolism after SLE exacerbation and an acute viral respiratory infection. In the iberdomide groups, seven AEs led to withdrawal by >1 patient (neutropenia [n = 4] and rash [n = 3]), and in the placebo group, two patients withdrew due to herpes zoster infection.
Table 3. Adverse events during the intervention*
|
AE, adverse event. |
||||
|
Event, % |
Iberdomide |
Iberdomide |
Iberdomide |
Placebo |
|---|---|---|---|---|
|
Any AE |
78 |
78 |
74 |
65 |
|
Any intervention-related AE |
40 |
44 |
33 |
29 |
|
Any serious AE |
7 |
5 |
7 |
8 |
|
Any severe AE |
1 |
5 |
7 |
6 |
|
Any AE leading to interruption of intervention |
28 |
17 |
24 |
18 |
|
Any AE leading to the withdrawal of intervention |
5 |
13 |
5 |
7 |
|
Death |
0 |
0 |
0 |
1 |
|
AE with a frequency of ≥5%† |
|
|
|
|
|
Urinary tract |
10 |
16 |
5 |
4 |
|
Upper respiratory |
12 |
9 |
7 |
5 |
|
Neutropenia |
11 |
7 |
5 |
2 |
|
Influenza |
6 |
5 |
7 |
4 |
|
Nasopharyngitis |
9 |
1 |
7 |
1 |
|
Leukopenia |
6 |
4 |
2 |
1 |
|
Diarrhea |
4 |
2 |
7 |
0 |
|
Sinusitis |
6 |
0 |
2 |
1 |
|
Headache |
0 |
0 |
5 |
6 |
Conclusion
The study showed the promising efficacy and safety of iberdomide in patients with SLE. At 24 weeks, significantly higher SRI-4 response was observed with the highest dose of iberdomide (0.45 mg) compared with placebo, but not with the lower doses. The findings are preliminary and further long-term trials are required to confirm the clinical efficacy and safety of iberdomide in patients with SLE.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

