All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Immunomodulatory mechanisms of anifrolumab: A transcriptomic and proteomic analysis of phase III trials
Anifrolumab, a type I interferon receptor 1 (IFNAR1) blocking antibody, is approved for treatment of adults with moderate to severe systemic lupus erythematosus (SLE). It acts by blocking type I interferon (IFN) signaling that plays a key role in the pathogenesis of SLE.
Below, we summarize an article published by Baker et al. in Annals of the Rheumatic Diseases investigating the immunomodulatory mechanisms underlying IFNAR1 blockade by anifrolumab using longitudinal transcriptomic and proteomic analyses of the phase III TULIP-1 and TULIP-2 trials.
Methods
- Patients with moderate-to-severe SLE receiving intravenous anifrolumab or placebo alongside standard therapy in the TULIP-1 (NCT02446912) and TULIP-2 (NCT02446899) trials were assessed.
- Whole-blood expression of 18,017 genes and 184 plasma protein analytes were assessed.
- Treatment groups were compared for gene counts and protein levels via gene set enrichment analysis using MetaBase pathway analysis, blood transcriptome modules, in silico deconvolution of RNA sequencing, and longitudinal linear mixed effect models.
Key findings1
- A total of 726 patients were analyzed.
- 502 patients comprised the transcriptomic dataset (244 received anifrolumab and 258 received placebo).
- 256 patients from TULIP-1 comprised the proteomics dataset (124 patients received anifrolumab and 132 received placebo).
Differentially expressed genes
- At Week 24 and 52, anifrolumab resulted in downregulation of 1,681 and 2,092 genes, respectively, and upregulation of 1,170 and 1,645 genes, respectively vs placebo.
- The most significantly downregulated genes at Weeks 24 and 52 are shown in Figure 1.
Figure 1. Most significantly downregulated genes by anifrolumab vs placebo*
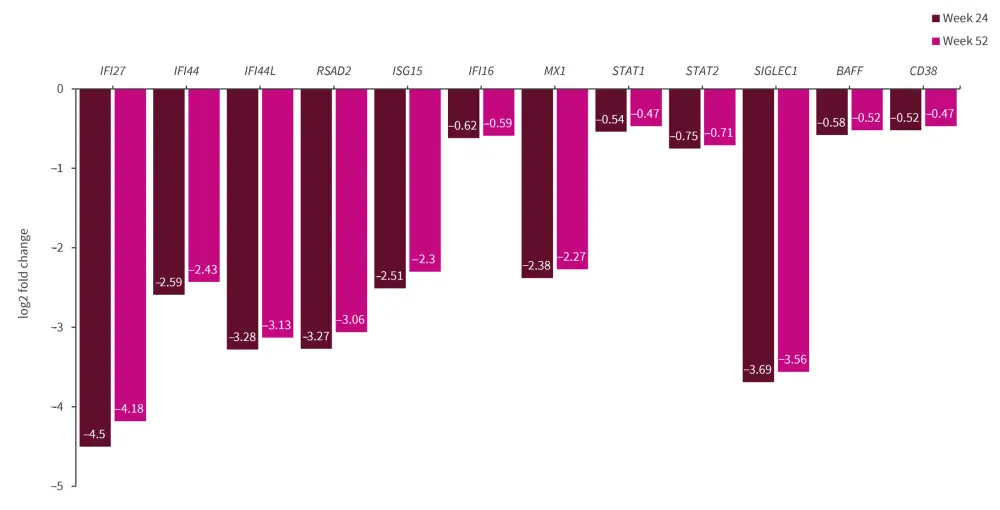
*Data from Baker, et al.1
Differentially regulated pathways
- Several MetaBase intracellular signaling pathways were downregulated including
- IFN-α/-β29 via:
- Janus kinases/signal transducer and activator of transcription (normalized enrichment score [NES] −2.84);
- phosphoinositide 3-kinases and nuclear factor kappa light chain enhancer of activated B cells (NES −2.13); and
- mitogen-activated protein kinase pathways (NES −2.21).
- IFN-γ29 via:
- IFN-γ mediated apoptosis induction and inhibition of proliferation (NES −2.32)
- IFN-γ actions on extracellular matrix and cell differentiation (NES −2.13).
- NETosis-associated pathways (NES −2.21).
- IFN-α/-β29 via:
- Several MetaBase pathways associated with protein synthesis were upregulated, including regulation of translation initiation (NES 3.59).
Blood transcriptome modules
- Anifrolumab downregulated modules associated with IFN activity compared with placebo at Week 52 (false discovery rate p value [PFDR] ≤0.01).
- Anifrolumab also downregulated modules for non-IFN pathways including modules for antigen presentation (NES −2.03), neutrophils (NES −1.88), and monocytes (NES −1.86).
- Several modules were upregulated including those associated with lymphocytes and T cells, as well as protein modification and synthesis (PFDR ≤0.01).
Proteins longitudinally modulated
- Anifrolumab significantly downregulated 34 and upregulated 7 proteins (PFDR ≤0.05, each).
| Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

