All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Impact of medium-to-high glucocorticoid dose on the risk of bloodstream infections in patients with SLE
For patients with systemic lupus erythematosus (SLE), severe infections such as bloodstream infections (BSIs) pose a significant risk of morbidity and mortality. Treatment with immunosuppressive agents, such as glucocorticoids, can increase the risk of BSIs, with studies also suggesting an association with increased risk of disease flare.1
However, the relationship between BSI, immunosuppressants, and disease activity remains to be thoroughly investigated. A recent study by Kim et al.1 explored the incidence rate of BSIs and associated risk factors in patients with SLE treated with medium to high doses of glucocorticoids.
Study design
This retrospective single-center cohort study identified patients with SLE treated with ≥15 mg/day of prednisolone or equivalent for more than 4 weeks (defined as a treatment episode). Treatment episodes >1 year apart were counted as separate episodes.
Patients were classified as having SLE if they had ≥4 1997 American College of Rheumatology revised classification criteria for SLE at time of diagnosis.
The main study endpoint was incidence of BSI. Other endpoints included incidence rate stratified by disease activity, estimated with Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K), and the initial dose of glucocorticoid (GC) at the start of the study. Clinical factors significantly associated with BSI were also examined.
Of note, confirmation of a pathogen in the blood with signs or symptoms of systemic inflammatory response syndrome were also required for a BSI diagnosis.
Results
Baseline characteristics
In this study, 83.4% of patients were female and the average age was 37 years. Over half of the patients included had low complement levels, high anti-double stranded DNA and proteinuria at baseline. Over a third of patients received ≥60 mg/day of prednisolone at the index date (Table 1).
Table 1. Baseline patient characteristics*
|
ds, double-stranded; HCQ, hydroxychloroquine; SD, standard deviation; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000; TMP/SMX, trimethoprim-sulfamethoxazole. |
|
|
Clinical features, % (unless otherwise stated) |
Treatment |
|---|---|
|
Mean age (±SD), years |
37.0 (±12.7) |
|
Female |
83.4 |
|
Mean disease duration (±SD), years |
5.5±4.8 |
|
Mean SLEDAI-2K (±SD), n† |
10.4±6.3 |
|
SLEDAI-2K ≥20 |
7.8 |
|
Proteinuria‡ |
57.6 |
|
Low complement§ |
51.5 |
|
High anti-dsDNA‖ |
61.9 |
|
Leukopenia¶ |
9.8 |
|
Thrombocytopenia# |
12.0 |
|
Azotemia** |
23.9 |
|
Positive anti-phospholipid antibodies†† |
34.1 |
|
≥60 mg/day of prednisolone (including pulse treatment) at the index date |
35.1 |
|
Mean cumulative glucocorticoid dose during previous 6 months (±SD)† |
1,442.6 (±1,699.7) |
|
Concomitant immunosuppressive treatment |
|
|
Glucocorticoid pulse treatment |
19.0 |
|
HCQ |
36.8 |
|
Oral cyclophosphamide |
16.9 |
|
Cyclophosphamide pulse treatment |
10.8 |
|
Mycophenolate mofetil |
15.6 |
|
Azathioprine |
11.5 |
|
Methotrexate |
2.9 |
|
Cyclosporine |
4.8 |
|
Rituximab |
0.6 |
|
TMP/SMX prophylaxis |
12.9 |
In total, 1,109 treatment episodes were recorded in 612 patients. Thirty cases of BSI were recorded, with an incidence rate of 2.78 (95% confidence interval [CI], 1.88–3.97) per 100 person-years; the characteristics of this group are shown in Table 2. There was an average of 152.8 (standard deviation [SD], ±87.0) days between index date and BSI being recorded. The mean daily dose of GCs was 44.8 (SD, ±60.9 mg). SLE disease flares were recorded in six cases with an increased dose of GCs before BSI.
Out of the 30 patients with a BSI, 16.7% died as a result of the infection compared with 0.8% of patients with no BSI. The incidence rate ratio of 1 year mortality and ICU admission were elevated in treatment episodes with BSI compared with those without at 9.59 (95% CI, 7.33–52.44) and 34.93 (95% CI, 17.49–69.79), respectively.
Table 2. Clinical characteristics and outcomes at the time of BSI*
|
BSI, blood stream infection; ds-DNA, double stranded; SD, standard deviation. |
|
|
Features |
N = 30 |
|---|---|
|
Mean age (±SD), years |
47.9 (±15.7) |
|
Female, % |
83.3 |
|
Mean disease duration (±SD), years |
7.7 (±5.3) |
|
Comorbidities, % |
|
|
Diabetes |
18.5 |
|
Cerebrovascular disease |
14.8 |
|
Chronic kidney disease |
11.1 |
|
End stage renal disease on dialysis |
11.1 |
|
Valvular heart disease |
11.1 |
|
Interstitial lung disease |
11.1 |
|
Mean total leukocyte count (SD), number/uL |
7,400 (4,100) |
|
Low complement, % |
63.3 |
|
Mean anti-ds-DNA antibodies (SD), IU/mL |
145.1 (365.9) |
|
Mean prednisolone dose at BSI (±SD), mg |
44.8 (±60.9) |
|
Mean time from baseline to BSI (±SD), days |
152.8±87.0 |
|
Mean cumulative glucocorticoid dose during the period between the baseline and BSI (±SD), mg |
7,155.0 (±4,184.6) |
|
Mean cumulative glucocorticoid dose during previous 6 months (SD), mg† |
2,699.1±2,691.9 |
|
Outcomes of BSI |
|
|
Mechanical ventilation, % |
50.0 |
|
BSI-related death, % |
16.7 |
The most common infectious agent associated with BSIs was E. coli and the most frequent site of infection was the respiratory tract (Figure 1).
Figure 1. Most common infectious agents and site of initial infection*
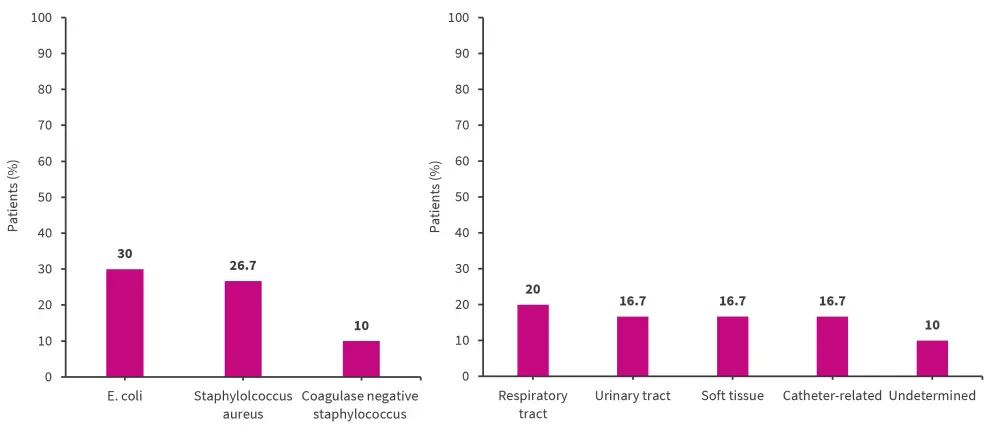
*Adapted from Kim, et al.1
Clinical risk factors
The incidence rate ratio (IRR) of BSI in patients treated with ≥60 mg/day of prednisolone or equivalent at baseline was significantly increased at 5.01 (95% CI, 3.01–7.82) per 100 person-years compared with the group treated with 15–30 mg (p = 0.002) or 30–60 mg (p = 0.079). An increase in disease activity also significantly raised the IRR of BSIs. Patients with SLEDAI-2K scores ≥20 showed a significantly increased IRR compared with all other SLEDAI-2K index score groups (0–5, 6–10 and 11–19; p < 0.001).
Multivariate analysis revealed several factors significantly associated with increased BSI risk, including age, azotemia, disease activity, baseline prednisolone dose ≥60 mg/day, and higher mean daily GC dose (Table 3). The Pearson’s correlation coefficient between SLEDAI-2K and initial GC dose was not relevant at 0.199. In univariate analysis, prophylactic use of trimethoprim-sulfamethoxazole was not associated with a change in BSI incidence rate; however, following adjustments for baseline GC dose and SLEDAI-2K index, trimethoprim-sulfamethoxazole was associated with a significant decrease in BSI risk (adjusted IRR, 0.42; 95% CI, 0.18–0.99).
Table 3. Clinical factors significantly associated with incidence of BSI*
|
CI, confidence interval; IRR, incidence rate ratio; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000. *Adapted from Kim, et al.1 ‡Defined as estimated glomerular filtration rate <60 mL/min/1.73 m2. |
||||
|
Clinical factors |
Univariable analysis |
Multivariable analysis† |
||
|---|---|---|---|---|
|
IRR (95% CI) |
p-value |
IRR (95% CI) |
p-value |
|
|
Age, years |
1.06 (1.03–1.09) |
<0.001 |
1.05 (1.02–1.08) |
<0.001 |
|
Diabetes |
4.20 (1.86–9.52) |
0.001 |
1.21 (0.55–2.66) |
0.643 |
|
SLEDAI-2K ≥20 |
7.15 (3.14–16.26) |
<0.001 |
4.66 (2.17–10.00) |
<0.001 |
|
Azotemia‡ |
7.59 (3.43–16.80) |
<0.001 |
4.95 (2.41–10.18) |
<0.001 |
|
Baseline prednisolone |
3.24 (1.45–7.23) |
0.009 |
2.42 (1.11–5.32) |
0.027 |
|
Mean daily dose of glucocorticoid |
3.16 (1.46–6.86) |
0.004 |
2.13 (1.03–4.40) |
0.042 |
|
Concomitant cyclophosphamide |
4.08 (1.77–9.40) |
0.001 |
1.90 (0.81–4.47) |
0.139 |
Limitations
The authors noted several limitations, including the lack of complete results for all patients due to retrospective nature of the study. The overall number of BSI cases was relatively low, which may impact data on risk factors, and the administered GC dose throughout the study was not fixed, which may have influenced the risk of BSI.
Conclusion
A BSI incidence rate of 2.78 (95% CI, 1.88–3.97) per 100 person years was recorded, which is significantly increased compared with the general public. In this study, moderate-to-high doses of GC (≥60 mg/day) and high baseline disease activity were associated with an increased risk of BSI. The most frequent infectious agent causing a BSI was found to be E. coli and the most common focus of infection was the respiratory tract. The mortality rate of patients with SLE and BSI remains elevated compared to those without a BSI. This highlights the ongoing need for effective surveillance and prophylactic strategies to prevent BSIs in patients with SLE.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

