All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Litifilimab in patients with cutaneous lupus erythematosus
Litifilimab, previously known as BIIB059, is a humanized monoclonal antibody against the blood dendritic cell antigen 2 (BDCA2) receptor.1 The BDCA2 antigen is exclusively expressed on plasmacytoid dendritic cells, which are thought to play a role in the pathogenesis of cutaneous lupus erythematosus (CLE).1
To assess whether litifilimab is effective for the treatment of systemic lupus erythematosus (SLE) and CLE, a two-part phase II study (LILAC; NCT02847598) was performed.1 Part A investigated patients with SLE who had active joint and skin involvement. Whereas Part B examined patients with moderate-to-severe CLE with or without SLE, with the findings reported by Werth, et al.1 and summarized here.
Study design
At first, Part B included 31 patients who were randomized 2:1 to receive 450 mg litifilimab or placebo (protocol 1). However, to examine a range of doses, the protocol was modified to include two more doses of litifilimab at 50 mg and 150 mg.
The modified protocol for Part B of the LILAC phase II study included 132 patients who were randomly assigned to be given either 50 mg, 150 mg, or 450 mg of litifilimab or placebo in a 1:1:1:1 ratio at Weeks 0, 2, 4, 8, 12, and 16. The primary endpoint was the percentage change from baseline in the Cutaneous Lupus Erythematosus Disease Area and Severity Index-Activity (CLASI-A) score at Week 16 to evaluate the dose–response relationship. This score measures the extent of skin involvement (erythema and scaling) and severity, with a score of 0−70 in 13 skin regions and higher scores indicating more severe disease.
Secondary endpoints:
- A decrease of ≥50% from baseline in the CLASI-A score (CLASI-50) at Weeks 12 and 16
- The percentage change from baseline in the CLASI-A score at Week 12
- Reductions from baseline in the CLASI-A score of ≥4 points at Weeks 12 and 16 and of ≥7 points at Weeks 12 and 16
- Changes in pharmacokinetic measures
Eligibility criteria:
- Adults aged 18−75 years with active CLE (CLASI-A score ≥8) confirmed by biopsy
- Either active subacute CLE (CLASI-A erythema score of ≥2) or chronic CLE (minimum CLASI-A erythema score of ≥2 and a score of ≥1 on the CLASI-Damage scarring scale, which measures scarring, atrophy, and panniculitis on a scale of 0–2)
- Received topical agents or antimalarials but had insufficient response or intolerable side effects
Results
The number of patients that received each dose of litifilimab or placebo is shown in Table 1 along with the baseline characteristics. Out of these patients, 9% did not complete their assigned treatment regimen; 8 due to adverse events (3 in the 50 mg group, 1 in the 150 mg group, and 4 in the 450 mg group) and 4 following withdrawals of consent.
In general, patient characteristics were balanced between groups, with a few exceptions, including sex, race, and oral glucocorticoid dose.
Table 1. Baseline patient characteristics*
|
CLASI-A, Cutaneous Lupus Erythematosus Disease Area and Severity Index-Activity; CLE, cutaneous lupus erythematosus; GC, glucocorticoid; SLE, systemic lupus erythematosus; SLEDAI 2K, SLE Disease Activity Index 2000. |
||||
|
Characteristic |
Litifilimab |
Litifilimab |
Litifilimab |
Placebo |
|---|---|---|---|---|
|
Age, years |
43.3 ± 15.3 |
43.6 ± 12.1 |
44.0 ± 12.6 |
43.4 ± 11.6 |
|
Female, % |
77 |
80 |
75 |
91 |
|
Disease duration, year |
6.9 ± 10.9 |
8.4 ± 7.6 |
10.2 ± 8.9 |
8.8 ± 7.7 |
|
Race/ethnicity, %† |
|
|
|
|
|
Asian |
27 |
24 |
35 |
42 |
|
White |
15 |
24 |
27 |
27 |
|
African American or Black |
19 |
8 |
10 |
6 |
|
Hispanic or Latino |
19 |
4 |
8 |
9 |
|
Other |
12 |
0 |
6 |
0 |
|
Not reported |
27 |
44 |
21 |
24 |
|
CLASI-A score |
15.2 ± 8.8 |
18.4 ± 8.7 |
16.5 ± 8.8 |
16.5 ± 8.5 |
|
CLASI-A score >10, % |
69 |
80 |
71 |
67 |
|
CLE subtype‡ |
|
|
|
|
|
Acute |
4 |
4 |
0 |
3 |
|
Subacute |
31 |
44 |
31 |
33 |
|
Chronic |
73 |
68 |
69 |
70 |
|
SLE, % |
42 |
48 |
42 |
42 |
|
SLEDAI 2K score |
6.5 ± 3.1 |
6.7 ± 3.4 |
6.2 ± 2.6 |
7.3 ± 2.6 |
|
Receiving oral GC, % |
38 |
40 |
50 |
58 |
|
Daily oral GC dose, mg |
10.9 ± 4.0 |
8.2 ± 2.8 |
7.3 ± 3.7 |
7.0 ± 3.2 |
|
Receiving medication for CLE, SLE, or both |
88 |
84 |
94 |
88 |
|
Antimalarial agent, % |
78 |
86 |
82 |
69 |
|
Antimalarial + GC, % |
48 |
52 |
51 |
55 |
|
Azathioprine, % |
9 |
10 |
4 |
7 |
|
Methotrexate, % |
9 |
5 |
4 |
3 |
|
Mycophenolate, % |
0 |
10 |
9 |
0 |
Primary endpoint
The least-squares mean (LSM) percentage change in CLASI-A score for the placebo and litifilimab dose groups is shown in Figure 1. All litifilimab doses showed a greater change from the baseline compared with the placebo group, with 150 mg litifilimab at Week 16 demonstrating the largest deviation at −33.4% versus the placebo (95% confidence interval [CI], −52.7 to −14.1) followed by −28.0 percentage points (95% CI, −44.6 to −11.4) in the 450 mg group and −24.3 percentage points (95% CI, −43.7 to −4.9) in the 50 mg group .
Figure 1. Primary endpoint*
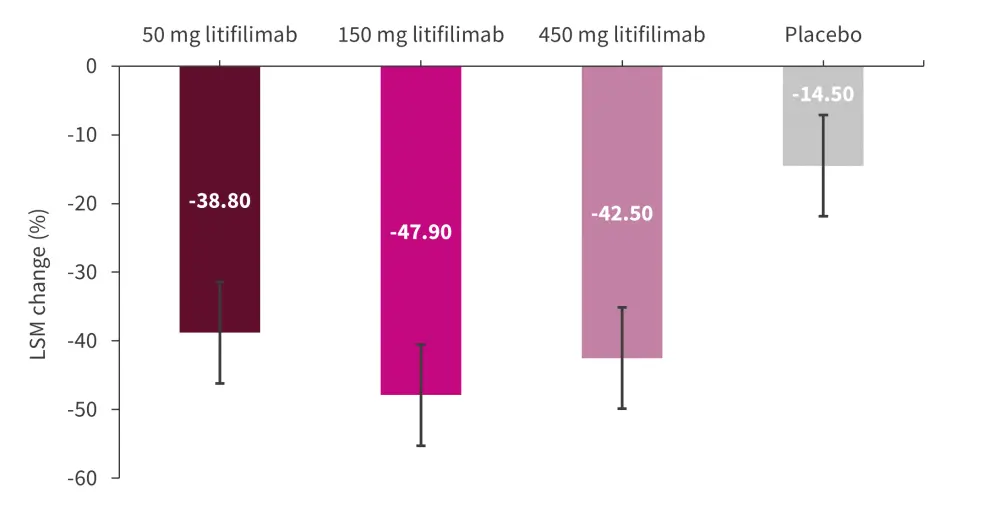
LSM, least-squares mean.
*Data from Werth, et al.1
The secondary endpoints for this trial were investigated, although the authors stated that this study was not powered to assess the secondary endpoints. The LSM differences for all litifilimab doses versus placebo had a 95% CI of zero for the majority of the secondary endpoints, excluding CLASI-50 at Week 12, a CLASI-50 response at Week 16 for the 450 mg litifilimab dose only, and a 4‑point or greater decrease in CLASI-A scores at Weeks 12 and 16 for the 150 mg litifilimab dose only. No inferences can be made from these data as a result, as the analysis did not compensate to exclude zero in these cases.
Pharmacokinetics and serological analysis
Serum concentrations of litifilimab were seen to increase in a dose-dependent manner.
After 16 weeks, no clinically relevant changes in the level of C3 or C4 complement proteins were observed, although small differences were noted in SLE-related autoantibody titers, erythrocyte sedimentation rate, and C-reactive protein levels.
Safety
Overall, 72% of patients who received litifilimab (any dose) and 67% of patients treated with the placebo experienced an adverse event (AE; Figure 2). There were no fatal AEs recorded in this trial and pooled serious AEs were only 7% for the patients treated with litifilimab compared with 9% for the placebo group. AEs leading to trial withdrawal occurred in one case, while AEs leading to treatment discontinuation were recorded in eight patients (three in the 50 mg group, one in the 150 mg group, and four in the 450 mg group).
Figure 2. Adverse events*
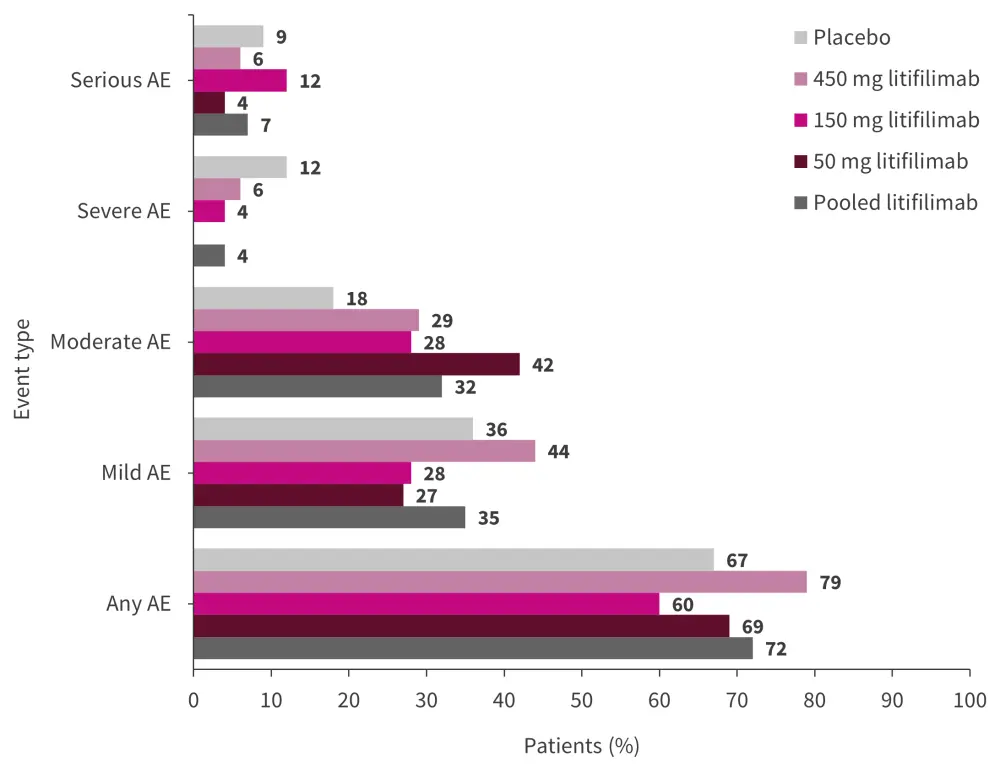
AE, adverse event.
*Data from Werth, et al.1
The most common AEs recorded were nasopharyngitis, headaches, SLE, injection-site erythema, and pruritis (Figure 3). In the pooled litifilimab group (n = 99), 5% of patients developed influenza, 3% had oral herpes infection, and 2% had a viral upper respiratory tract infection. A case of herpes zoster meningitis was recorded in the 50 mg litifilimab group at ~4 months after the last litifilimab dose. The placebo group recorded two cases of systemic viral infection and one herpes simplex infection.
Figure 3. Adverse events in ≥5% of patients in the litifilimab and placebo groups*
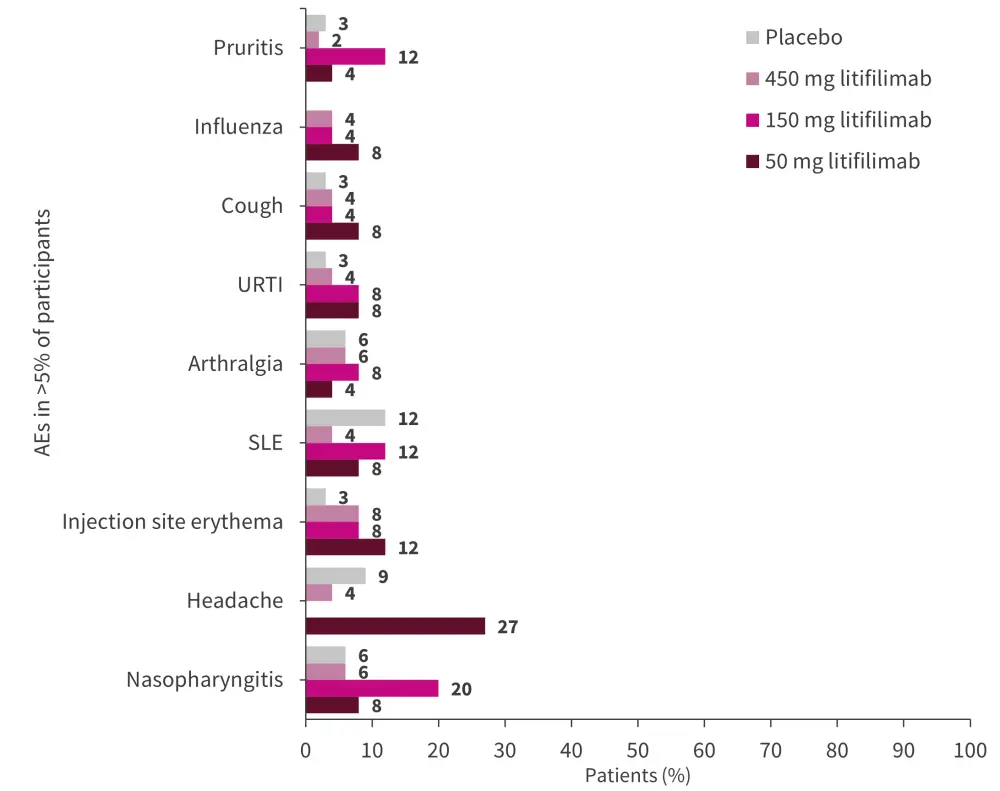
SLE, systemic lupus erythematosus; URTI, upper respiratory tract infection.
*Data from Werth, et al.1
Conclusion
This trial of litifilimab in patients with CLE met its primary endpoint of decreased CLASI-A score from baseline at 16 weeks and showed a significant dose–response relationship compared with the placebo group. The safety analysis highlighted that there were cases of herpes zoster and one case of herpes zoster meningitis that occurred in patients treated with litifilimab. Further studies with a larger cohort in a longer follow-up will be required to fully assess the safety and efficacy of litifilimab in patients with CLE along with the Part A trial results in SLE.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

