All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
SLE disease activity at conception, during pregnancy, and after birth
Do you know... According to a study by Radin et al., for how long will women with SLE in remission at conception breastfeed, compared with a median of 5.9 months in patients with active SLE?
Introduction
Despite advances in our understanding of the clinical course and prognosis of systemic lupus erythematosus (SLE), conception and pregnancy remain a challenging decision for women with SLE considering having children. During pregnancy, both maternal and fetal SLE complications may occur, with SLE flares occurring in approximately 2–68% of pregnancies based on data from current literature.1
The unpredictable disease course and occurrence of flares during pregnancy can carry significant burden and comorbidity for women of childbearing age, leading to anxiety and concerns for family planning.
An association between sex hormones and SLE activity during pregnancy is recognized, in particular considering hormonal changes in the peri-partum and post-partum period, including breastfeeding.1 However, limited knowledge or evidence currently exists around disease activity in the immediate (up to 6 months after delivery) and extended postpartum period, including predicting factors that may influence maternal and neonatal outcomes after childbirth.
The Lupus Hub is pleased to present recent data on this topic from Radin et al.1 published in Seminars in Arthritis and Rheumatism in 2022. This retrospective cohort study explored SLE course and prognosis in pregnant women to identify clinical parameters affecting disease activity in this patient cohort up to 2 years postpartum.
Study design
This multicenter, retrospective cohort study included women diagnosed with SLE with a first birth treated between 2000 and 2019 at eight centers in Italy, Brazil, Spain, Japan, and Argentina.
Data was collected from clinical charts and medical records, with biological and clinical data including:
- anti-nuclear antibodies
- extractable nuclear antigens antibodies
- anti-double stranded DNA
- antiphospholipid antibodies
- full blood count, creatinine, liver enzymes, complement levels, serum protein electrophoresis, immunoglobulins, and electrolytes
Comorbidities, including cardiovascular and thrombosis risk, were also recorded. All patients had a diagnosis of SLE, their first birth after 2000, and follow-up of at least 2 years after birth.
Results1
In total, 116 women were included in this retrospective study. Mean age at conception was 29.9 years (Standard Deviation [SD], ±5.8 years), with participants identified as having various stages of disease activity (scored using the Systemic Lupus Erythematosus Disease Activity Index 2000 [SLEDAI-2K])2 as follows:
- 41.2% were defined as being in remission
- 21.8% had very low disease activity
- 20.2% had low disease activity
- 16.8% no remission
All patients were positive for anti-nuclear antibodies, with 74.8% of patients presenting with positive anti-double stranded DNA (anti-dsDNA)-antibodies at diagnosis. Further diagnostic, clinical, and demographic data can be seen in Table 1.
Table 1. Patient characteristics*
|
aGAPSS, Adjusted Global Anti-Phospholipid Syndrome Score; ANA, anti-nuclear antibody; APS, antiphospholipid syndrome; dsDNA, double stranded DNA; ENA, extractable nuclear-antigen antibody; Ig, immunoglobulin; La/SSB, Sjögren's syndrome-related antigen B; RNP, ribonucleoprotein; Ro/SSA, Sjögren's syndrome-related antigen A; Scl 70, scleroderma 70 kD antigen (topoisomerase I); SD, standard deviation; SLE, systemic lupus erythematosus; Sm, Smith antibodies; U1RNP, U1 ribonucleoprotein. |
|
|
Parameter, % (unless otherwise stated) |
|
|---|---|
|
Mean age at inclusion (SD), years |
37.6 (±7.36) |
|
Mean age at conception (SD), years |
29.85 (±5.75) |
|
Secondary diagnosis |
|
|
APS |
13.5 |
|
Sjögren syndrome |
8.4 |
|
Clinical SLE characteristics at diagnosis |
|
|
Acute cutaneous |
63.9 |
|
Malar rash |
48 |
|
Oral/nasal ulcers |
36.13 |
|
Non scarring alopecia |
34.5 |
|
Joint |
79.8 |
|
Serositis |
23.5 |
|
Renal |
41.2 |
|
Biopsy-proven lupus nephritis |
26.9 |
|
Hematological |
53.8 |
|
Leukopenia |
36.1 |
|
Thrombocytopenia |
21.9 |
|
Laboratory characteristics at diagnosis |
|
|
ANA |
100 |
|
anti-dsDNA |
74.8 |
|
ENA |
48.7 |
|
anti-Sm |
28.6 |
|
anti-Ro/SSA |
37 |
|
anti-RNP |
20.2 |
|
Antiphospholipid antibodies |
40.3 |
|
Lupus anticoagulant |
21.8 |
|
Anticardiolipin antibodies (IgG/IgM) |
28.6 |
|
Low complement |
64.7 |
|
Positive Direct Coombs test |
23.5 |
|
Comorbidities |
|
|
Arterial hypertension |
16 |
|
Hyperlipidemia |
6.7 |
|
Mean aGAPSS (±SD) |
2.97 (±3.6) |
|
Previous venous thrombosis |
6.7 |
|
Previous arterial thrombosis |
2.5 |
Pregnancy and birth data
Mean SLEDAI-2K of patients at conception of their first birth was 2.8 (SD, ±4.35). During pregnancy, 84.9% of patients received hydroxychloroquine, 55.5% received low-dose daily prednisolone (<7.5 mg daily), and 23.5% received medium- to high-dose daily prednisolone (>7.5 mg daily). Only two patients did not achieve a complete resolution of clinical symptoms within 3 months of pregnancy. Overall, 17 patients (14.3%) experienced a disease flare during pregnancy. Joint, renal, hematologic, and cutaneous were the most common domains of disease flare (Figure 1).
Figure 1. Relative distribution of SLE flares during pregnancy according to organ/system*
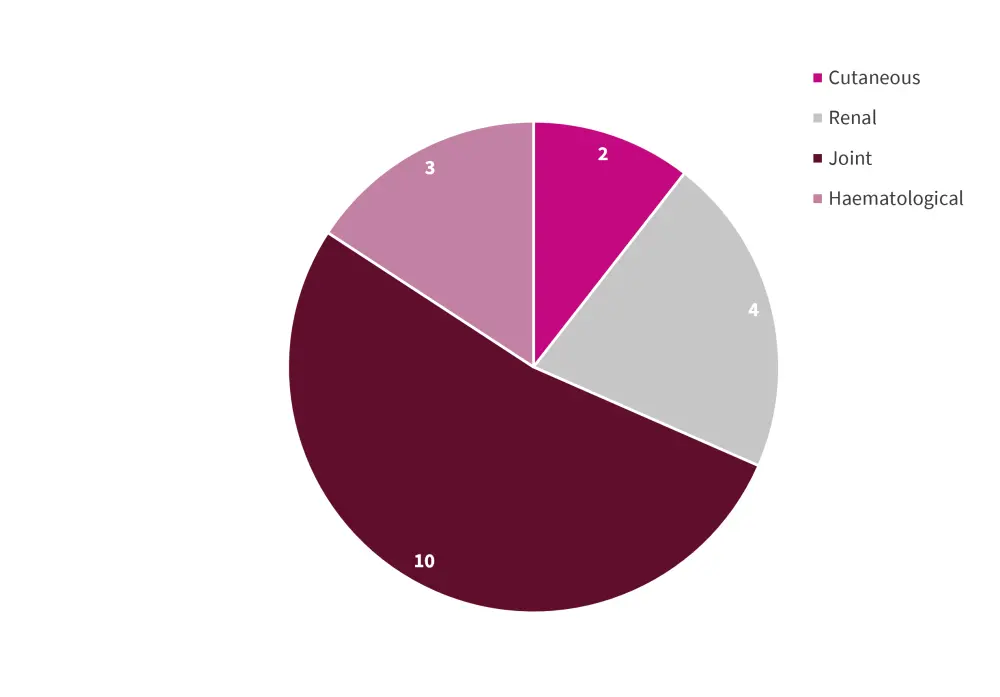
SLE, systemic lupus erythematosus.
*Adapted from Radin, et al.1
Pregnancy outcome and breastfeeding data can be seen in Table 2.
Table 2. Pregnancy and birth outcomes*
|
HELPP, hypertensions, elevated liver proteins, and low platelets; SD, standard deviation †Percentages are calculated from viable babies (total = 169). ‡Percentages are calculated from first birth (total = 119). §Exclusive breastfeeding was defined as feeding infants only breast milk. |
|
|
Outcomes, % (unless otherwise stated) |
|
|---|---|
|
Live births |
79 |
|
Miscarriages |
17.3 |
|
Stillbirths |
3.7 |
|
Maternal and fetal Complications |
|
|
Prematurity |
18.2 |
|
Pre-eclampsia |
10.7 |
|
HELLP syndrome |
1.9 |
|
Placental Infarction |
5.6 |
|
Breastfeeding (all pregnancies) |
|
|
Any breastfeeding |
64.5† |
|
Exclusive breastfeeding§ |
51.5† |
|
Breastfeeding (after first birth) |
|
|
Any breastfeeding |
73.1‡ |
|
Mean breastfeeding duration (±SD), months |
10.29 (±10.04) |
|
Exclusive breastfeeding§ |
52.1‡ |
|
Mean exclusive breastfeeding duration (±SD)§, |
6.07 (±6.0) |
Follow-up data at 2 years
During the follow-up period, 51.3% of patients experienced at least one SLE flare after birth, with a mean of 0.94 flares per patient (SD, ±1.1), and median time to first flare of 9 months (SD, ±6.3) from birth. Mean length of flare was 6.8 months, with a mean SLEDAI-2K score increase at first flare of 6.8 points (SD, ±4.3) compared with SLDEAI-2K at conception.
In 26.2% of patients, the first flare occurred in more than one organ or system. The distribution of most common first flare domains can be seen in in Figure 2, with 85 first flares happening in 61 patients.
Figure 2. First postpartum SLE flare domains according to organ/system*
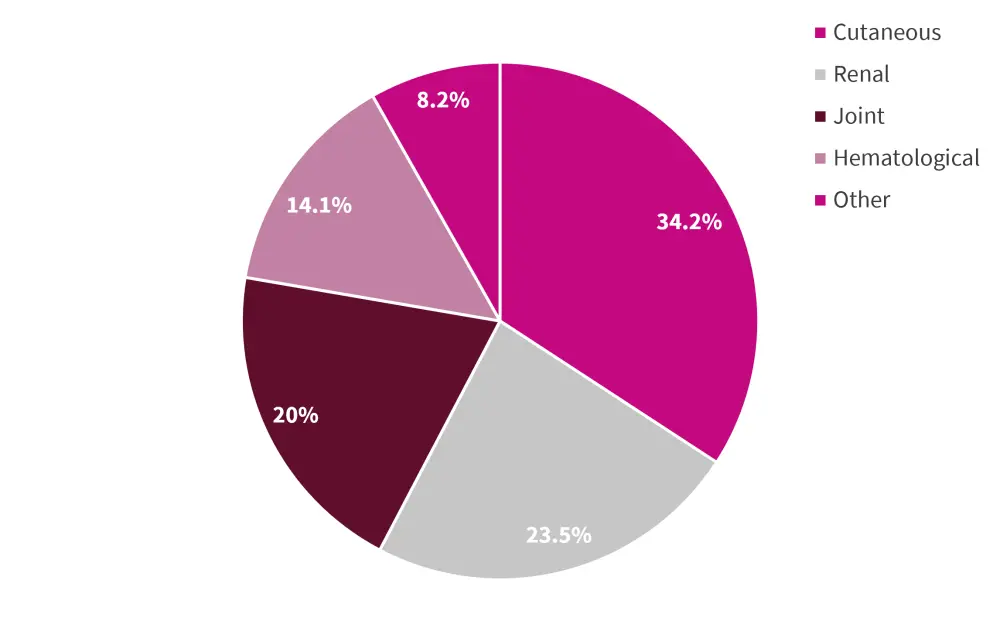
SLE, systemic lupus erythematosus.
*Adapted from Radin, et al.1
Patients in remission at conception had significantly lower rates of flares during follow-up compared with those who had disease activity at conception (36.7% vs 61.4%; p = 0.008). In addition, compared with other patients (n = 43), patients in remission at conception (n = 18) had
- a lower number of flares at follow-up (1.1, SD ±0.32 vs 1.5, SD ±0.7; p= 0.003);
- a lower cumulative duration of all flares (4.8 months; SD, ±3.1 months vs 8 months; SD, ±5.1 months; p = 0.004); and
- a longer duration of any breastfeeding (10.1 months; SD, ±11.8 months vs 5.9 months; SD, ±8.2 months; p = 0.024).
Notably, patients that presented with negative anti-dsDNA antibodies at conception had lower rate of SLE flares during follow-up compared with patients with positive anti-dsDNA antibodies at conception (67% vs 82%; p = 0.008).
Patients who experienced flares during pregnancy, when compared with those who did not, showed
- higher rates of flares during follow-up (76% vs 47%; p = 0.019);
- lower time to first flare postpartum (4.4 months; SD, ±2.3 months vs 10.3 months; SD, ±6.5; p < 0.001); and
- lower rates of exclusive breastfeeding (24% vs 57%; p=0.009).
Conclusion
Despite limitations related to the retrospective nature of the study and included patient cohort, this recent publication by Radin et al.1 demonstrated that being in remission at conception can positively influence SLE disease course, including long-term postpartum follow-up. When managing patients with SLE, pregnancy counselling is imperative to help control flares and disease activity prior to conception and to support active awareness and decision-making for women of childbearing age. Close monitoring during pregnancy is also essential, as flares during pregnancy are associated with higher rates of flares postpartum. Finally, this study shows that flares that happen both pre- and post-pregnancy may affect breastfeeding, suggesting that maternal disease activity has the potential to directly impact the mother, infant, and possibly the infant-mother bond.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

