All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Anti-dsDNA autoantibodies in the diagnosis and management of SLE: recommendations by an expert panel
Featured:
Anti-double stranded (ds) DNA autoantibodies are considered within the classification criteria of systemic lupus erythematosus (SLE) and are utilized to aid clinicians in effective diagnosis, monitoring disease activity and treatment response, and long-term disease management. However, clinical use of autoimmunity assays lacks standardization.
Below, we summarize recommendations by Rojo et al.,1 published in Autoimmunity reviews, for the use of anti-dsDNA autoantibodies in the diagnosis and management of SLE in routine practice.
Methods
Nine independent experts in autoimmunity from first-order Spanish institutions participated in a survey to identify the gaps, challenges, and areas for improvement in the diagnosis and management of SLE. Recommendations were formulated based on a non-systematic literature search, scientific evidence, the collective experience of the panel members, and their expert opinions.
Key findings
Testing for antinuclear antibodies (ANA) is considered an entry criterion in SLE classification. According to the European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR) 2019 classification criteria, ANA are considered positive at a titer ≥1:80 by an indirect immunofluorescence test on HEp-2 cells, with a specificity of 74.7%. In unselected populations, a 1:160 dilution is the cutoff, providing 86.2% specificity while maintaining 95.8% sensitivity. Cases with ANA titers between 1:80 and 1:160 may be further assessed by testing anti-extractable nuclear antigen antibodies, specifically anti-Ro autoantibodies.
Upon fulfilling the positive ANA entry criterion, the anti-dsDNA autoantibodies constitute the most prominent immunological criterion in the EULAR/ACR 2019 classification.
Anti-dsDNA is detected using following assays:
- Radioimmunoassay: Farr and Crithidia luciliae immunofluorescence test.
- Solid-phase assay (SPA): fluorometric enzyme-linked immunoassay, chemiluminescence immunoassay, enzyme-linked immune sorbent assay, and multiplex assay.
The expert panel recommends a double-screening strategy for testing anti-dsDNA, starting with a last-generation solid phase assay and confirming with the Farr and Crithidia luciliae immunofluorescence test. The algorithm for interpretation of lab tests is illustrated in Figure 1.
Figure 1. Algorithm for the interpretation of lab test results in the diagnosis of SLE*
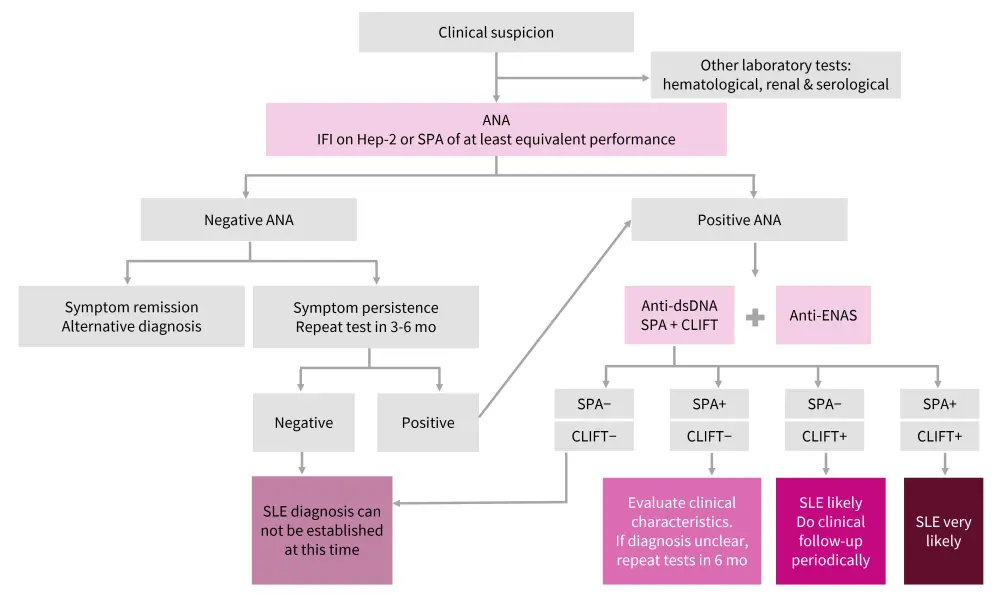
ANA, antinuclear autoantibody; CLIFT, Crithidia luciliae immunofluorescence test; dsDNA, double-stranded deoxyribonucleic acid; ENAS, extractable nuclear antigen; IFI, indirect immunofluorescence; mo, month; SLE, systemic lupus erythematosus; SPA, solid-phase assay.
*Adapted from Rojo, et al.1
The recommendations for optimizing routine clinical use of anti-dsDNA autoantibodies in the diagnosis and management of SLE are outlined in Figure 2.
Figure 2. Recommendations for utilizing anti-dsDNA autoantibodies for diagnosis and monitoring SLE*
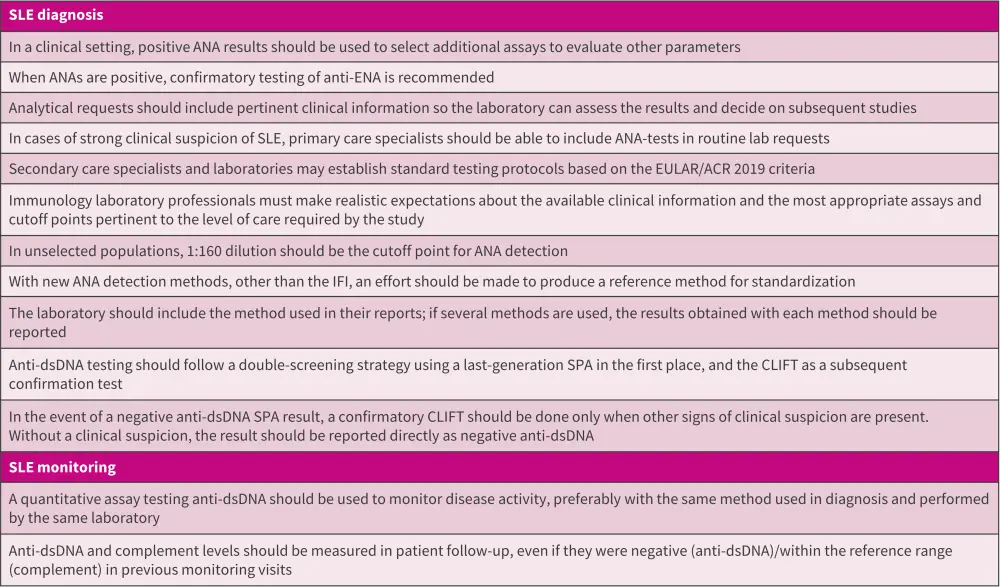
ANA, antinuclear autoantibodies; CLIFT, Crithidia luciliae immunofluorescence test; dsDNA, double-stranded deoxyribonucleic acid; ENAS, extractable nuclear antigens; IFI, indirect immunofluorescence; mo, month; SLE, systemic lupus erythematosus; SPA, solid-phase assay.
*Adapted from Rojo, et al.1
|
Key learnings |
|
The recommendations outline best practice for immunology laboratories to enhance the diagnosis and follow-up of SLE, emphasizing the optimized use of anti-dsDNA antibody testing, while also providing clinicians with a better understanding of methods used in the immunology laboratory. |
Question for expert

Expert opinion
Implementing these recommendations will require fluent communication between clinicians and laboratory professionals, with an improved understanding of the immunoassays used in ANA and anti-dsDNA determination allowing clinicians to make laboratory requests suited to their patient population and interpret ANA and anti-dsDNA results effectively.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Ricard Cervera
Ricard Cervera