All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Assessing laboratory markers as biomarkers for renal flare in patients with SLE
Patients with systemic lupus erythematosus (SLE) and lupus nephritis (LN) are at risk of developing severe renal complications, with up to 20% developing end-stage kidney disease within 10 years. The identification of biomarkers associated with renal flare could aid kidney monitoring, enabling the risk-stratification of patients and subsequent management changes.
During the American College of Rheumatology (ACR) annual meeting (ACR Convergence 2022), Gomez1 presented a post-hoc analysis of four phase III trials to assess laboratory markers as biomarkers for renal flare in patients with active SLE.
Study design
The study included four trials with similar designs:
- BLISS-52 (NCT00424476)
- BLISS-76 (NCT00410384)
- BLISS-SC (NCT01484496)
- BLISS-NEA (NCT01345253)
Together, these studies included 3,225 patients with SLE; the primary outcome was development of renal flare. Figure 1 outlines the biomarkers assessed.
Figure 1. Study design*
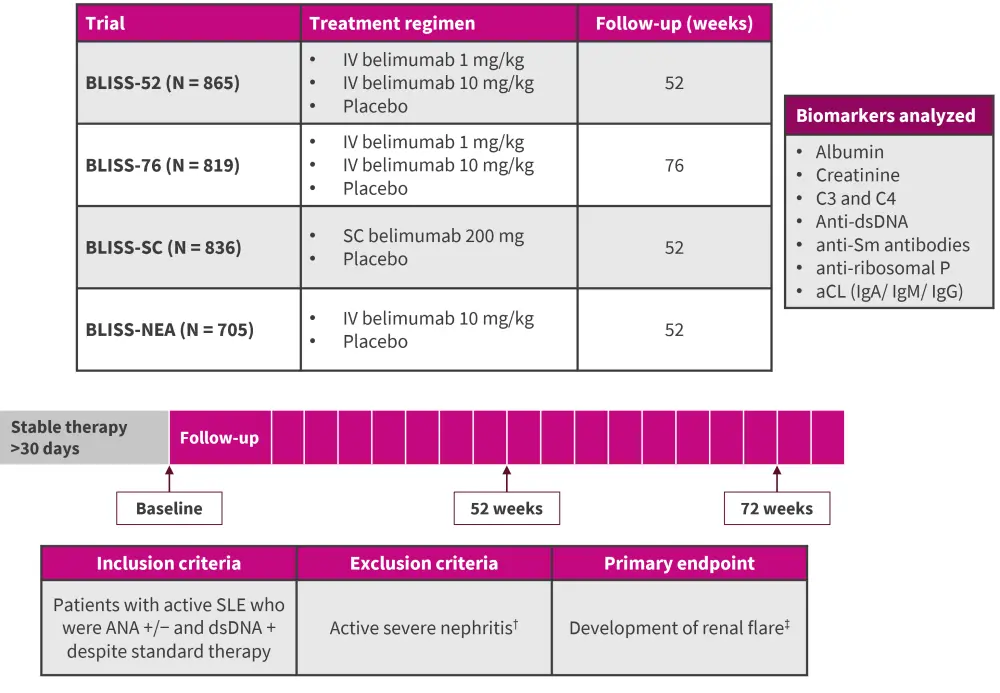
aCL, anticardiolipin; ANA, anti-nuclear antibody; ds, double-stranded; Sm, Smith; Ig, immunoglobulin; IV, intravenous; SC, subcutaneous; SLE, systematic lupus erythematosus.
*Adapted from Gomez.1
†Defined as: proteinuria >6 g/day, creatininemia >2.5 mg/mL, Active biopsy-proven lupus nephritis, or the requirement of hemodialysis 90 days prior to enrolment.
‡Defined as: increase in proteinuria, increase in serum creatinine, or new onset of hematuria of glomerular origin.
Results
During the analysis period, 192 patients had a renal flare. Patients in the renal flare group were younger; more frequently of Asian origin (p < 0.001); had significantly greater severity of disease activity, measured by Systemic Lupus Erythematosus Disease Activity Index 2000 score; and had experienced more renal involvement, measured by British Isles Lupus Assessment Group (BILAG) renal A-D index, compared with the whole cohort. However, fewer patients in the renal flare group had irreversible organ damage, measured as a Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI) >0, compared with those who did not experience renal flare.
Table 1. Baseline patient characteristics*
|
BILAG, British Isles Lupus Assessment Group; SD, standard deviation; SDI, Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index; SLE, systemic lupus erythematosus; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index. |
|||
|
Characteristic, % (unless stated otherwise) |
Renal flare |
No renal flare |
p value |
|---|---|---|---|
|
Mean age (SD), years |
32 (11) |
37 (12) |
<0.001 |
|
Female |
91 |
94 |
0.127 |
|
Ethnic origin |
|
|
|
|
Asian |
72 |
36 |
<0.001 |
|
African American |
5 |
7 |
0.157 |
|
Indigenous American |
8 |
14 |
0.021 |
|
Caucasian |
15 |
42 |
<0.001 |
|
Clinical characteristics |
|
|
|
|
Mean SLEDAI-2k (SD), n |
12 (4) |
10 (4) |
<0.001 |
|
Mean SLE duration (SD), years |
6 (6) |
6 (6) |
0.560 |
|
SDI >0 |
44 (23) |
1,102 (36) |
<0.001 |
|
BILAG renal A-D |
94 |
52 |
<0.001 |
|
Mean Prednisone dose (SD), |
14 (11) |
12 (9) |
0.003 |
|
Antimalarial use |
60 |
68 |
0.039 |
|
Belimumab use |
57 |
67 |
0.003 |
Survival analysis
The hazard ratio (HR) for the probability of renal failure was significantly increased following assessment of levels of C3 and all autoantibodies (Table 2).
Table 2. Biomarker analysis*
|
CI, confidence interval; ds, double-stranded; HR, hazard ratio; Sm, Smith. |
|||
|
Biomarker |
HR |
95% CI |
p value |
|---|---|---|---|
|
Low C3 |
2.9 |
2.1–4.1 |
<0.001 |
|
Low C4 |
1.2 |
0.9–1.6 |
0.147 |
|
Positive anti-dsDNA |
2.1 |
1.4–3.2 |
<0.001 |
|
Positive anti-Sm |
2.2 |
1.4–3.3 |
<0.001 |
|
Anti-ribosomal P |
2.3 |
1.5–3.6 |
<0.001 |
Following adjustments (for age, sex, ethnicity, body mass index, SDI, Systemic Lupus Erythematosus Disease Activity Index 2000 score, previous renal involvement, use of glucocorticoids, use of antimalarial agents, immunosuppression, and belimumab), low C3 and high proteinuria remained significant (p < 0.01 and p < 0.001, respectively) for indicating renal flare along with low levels of plasma albumin.
Subgroup analyses
The biomarkers of interest were also analyzed in a subgroup of patients who had BILAG A-D scores available (n = 1,761). In this subgroup analysis, only low C3 levels were significantly associated with an increased HR for the risk of renal flares (Table 3).
Table 3. Subgroup analysis of biomarkers for renal flare in patients with BILAG A-D score*
|
CI, confidence interval; ds, double-stranded; HR, hazard ratio; Sm, Smith. |
|||
|
Biomarker |
HR |
95% CI |
p value |
|---|---|---|---|
|
Low C3 |
2.2 |
1.6−3.1 |
<0.001 |
|
Positive anti-dsDNA |
1.4 |
0.9−2.1 |
0.126 |
|
Positive anti-Sm |
1.7 |
1.1−2.6 |
0.022 |
|
Anti-ribosomal P |
1.8 |
1.2−2.9 |
0.009 |
As belimumab is thought to improve low C3 and decrease anti-dsDNA, a subgroup analysis of patients treated with belimumab vs placebo was performed. In this analysis, the HR for baseline low C3 was 2.7 (range, 1.7−4.4) versus 3.3 (range, 2.1−5.0) in the belimumab arm. For positive presence of anti-dsDNA, the HR in the placebo group was 2.0 (1.2−3.5) compared with 2.3 (1.3−4.0). The investigators took these differences to be small enough to allow for the treatment arms to be examined together.
Conclusion
In this post-hoc analysis of four trials, low C3, hypoalbuminemia, and high levels of proteinuria were associated with increased risk of renal flares. Low C3 was also significantly associated with an increased risk of renal flares in a subgroup analysis of patients with available BILAG A-D scores. The results of this analysis suggest that these laboratory markers may be suitable as biomarkers for renal flares.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

