All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Baricitinib in SLE: Results from the phase III SLE-BRAVE trials
Outcomes in patients with systemic lupus erythematosus (SLE) remain poor. Conventional therapies include antimalarials, glucocorticoids, and immunosuppressants, with trials for new drugs often producing negative results and failing to progress beyond phase III trials.1
Baricitinib, an oral Janus kinase (JAK) 1 and 2 inhibitor, is already approved for several indications, including atopic dermatitis, alopecia, and rheumatoid arthritis; previous phase II trial of baricitinib have demonstrated improved SLE activity compared with placebo over 24 weeks. The SLE-BRAVE-I and -II trials (NCT03616912; NCT03616964) investigated the efficacy and safety of baricitinib in patients with SLE over 52 weeks.1,2
Trial design
SLE-BRAVE-I and SLE-BRAVE-II were both multicenter, randomized, double-blind phase III trials comparing baricitinib with placebo in patients with SLE.1,2 Eligible patients continued to receive their standard of care treatment alongside baricitinib or placebo.1,2 The study design is shown in Figure 1.
Figure 1. Trial design of SLE-BRAVE-I and SLE-BRAVE-II*
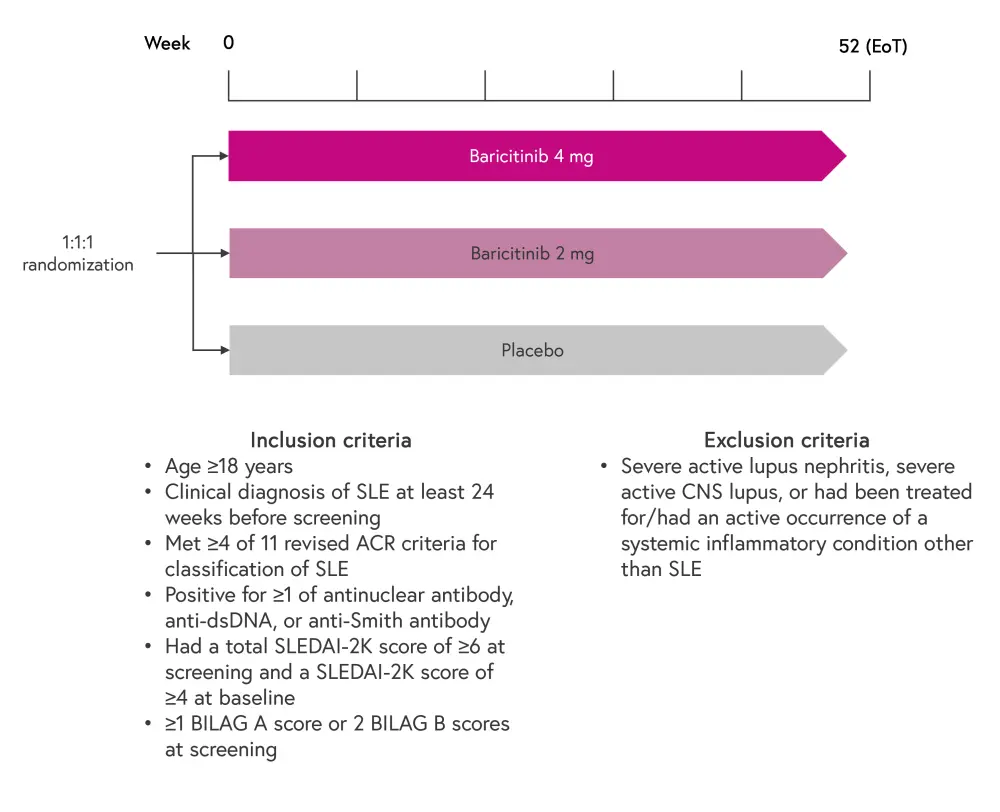
ACR, American College of Rheumatology; BILAG, British Isles Lupus Assessment Group; CNS, central nervous system; ds, double stranded; EoT, end of treatment; SLE, systemic lupus erythematosus; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index-2000; SRI-4, SLE Responder Index-4.
*Adapted from Morand, et al.1 and Petri, et al.2
The primary endpoint for both trials was the proportion of patients achieving a SLE Responder Index (SRI)-4 response at Week 52 in the baricitinib 4 mg group compared with placebo group.1,2 Secondary endpoints included:
- SRI-4 response at Week 24;
- SRI-4 response at Week 52 in patients receiving baricitinib 2 mg;
- Achievement of lupus low disease activity state at Week 52;
- 25% decrease in prednisone dose in patients receiving 7.5 mg or more at baseline;
- time to first severe flare;
- change from baseline in Worst Pain Numeric Rating Scale at Week 52; and
- change from baseline in Functional Assessment of Chronic Illness Therapy-Fatigue score at Week 52.
Adverse events were measured at each visit for both studies.
Results
In SLE-BRAVE-I, 769 patients were enrolled and 760 patients were included in the analyses.1 In SLE-BLAVE-II, 778 patients were enrolled and 775 were included in analyses.2 Key baseline patient characteristics are shown in Table 1.
Table 1. Baseline patient characteristics in SLE-BRAVE-I and -II*
|
NSAID, non-steroidal anti-inflammatory drug; SLE, systemic lupus erythematosus; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index-2000. |
||||||
|
Characteristic, % (unless otherwise stated) |
SLE-BRAVE-I |
SLE-BRAVE-II |
||||
|---|---|---|---|---|---|---|
|
Placebo |
Baricitinib 2mg |
Baricitinib 4mg |
Placebo |
Baricitinib 2mg |
Baricitinib 4mg |
|
|
Mean age, years |
42.0 |
42.9 |
41.5 |
43.5 |
42.8 |
42.2 |
|
Sex |
|
|
|
|
|
|
|
Male |
— |
— |
— |
6 |
6 |
5 |
|
Female |
94 |
93 |
94 |
94 |
94 |
95 |
|
Race |
|
|
|
|
|
|
|
Asian |
13 |
16 |
14 |
28 |
26 |
28 |
|
Black or African |
14 |
9 |
12 |
7 |
9 |
10 |
|
White |
67 |
69 |
71 |
58 |
59 |
55 |
|
Other†/multiple |
5 |
7 |
3 |
6 |
5 |
5 |
|
Mean time since onset of SLE, years |
9.4 |
9.2 |
8.8 |
9.0 |
8.7 |
8.5 |
|
Concomitant medications |
|
|
|
|
|
|
|
Glucocorticoids |
77 |
76 |
74 |
81 |
80 |
80 |
|
Mean |
9.8 |
10.4 |
10.1 |
8.8 |
9.6 |
9.8 |
|
Antimalarials |
84 |
74 |
82 |
82 |
82 |
83 |
|
Immuno-suppressants |
59 |
60 |
82 |
55 |
51 |
52 |
|
NSAID |
25 |
26 |
27 |
20 |
25 |
27 |
|
Mean SLEDAI-2K score |
10.1 |
10.3 |
10.0 |
10.1 |
10.1 |
10.1 |
Efficacy
The efficacy analysis was performed based on the intention-to-treat population.1,2 SLE-BRAVE-I did not to meet any of its major secondary endpoints,1 while SLE-BRAVE-II did not meet both its primary and major secondary endpoints.2 The proportion of patients meeting the primary outcome measures is shown in Figure 2, including the primary endpoint of SRI-4 at Week 52.
Figure 2. Primary outcomes in SLE-BRAVE-1 and SLE-BRAVE-II: A SRI-4 at Week 52, B reduction of ≥4 points from baseline in SLEDAI-2K score, C no new BILAG A and no more than one new BILAG B disease activity score, and D no worsening*
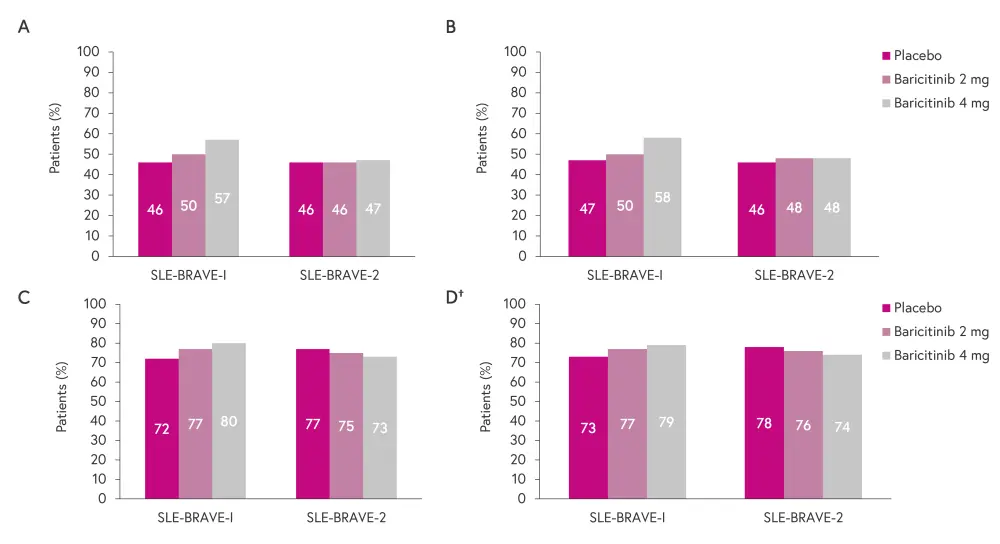
BILAG, British Isles Lupus Assessment Group; PGA, Physician Global Assessment; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index-2000; SRI-4, SLE Responder Index-4.
*Adapted from Morand, et al.1 and Petri, et al.2
†Defined as an increase of ≥0.3 points (10 mm) from baseline in PGA.
Safety
The incidence of adverse events and discontinuation was similar across treatment groups in both studies, as shown in Figure 3.1,2
Figure 3. Safety outcomes: incidence of A treatment-emergent adverse events, B serious adverse events, C infections, and D discontinuation due to adverse events*
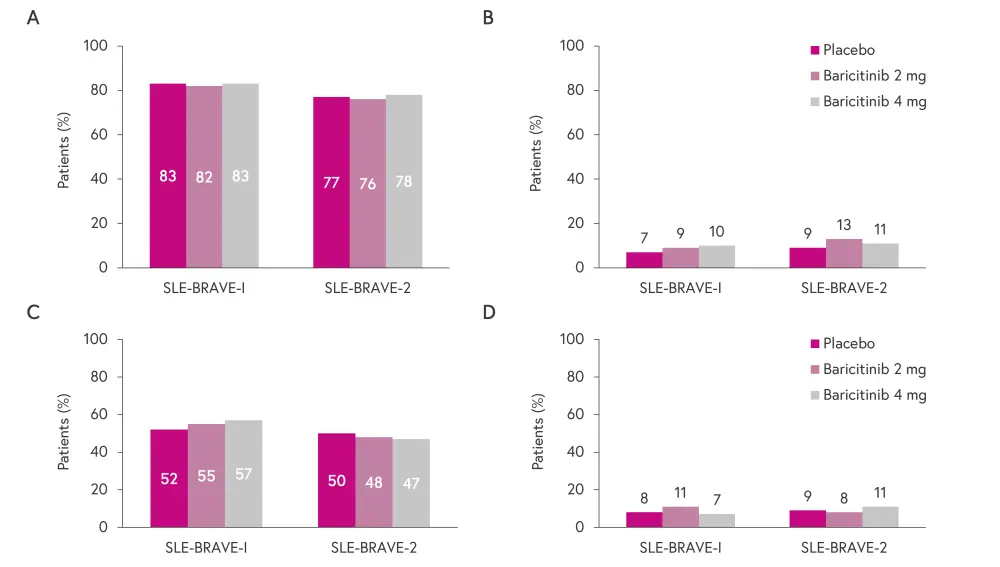
*Adapted from Morand, et al.1 and Petri, et al.2
Two deaths due to COVID-19 were reported in SLE-BRAVE-I, one in a patient receiving placebo and one receiving baricitinib 2 mg.1 In SLE-BRAVE-II, four deaths were reported in patients treated with baricitinib 4 mg, two due to myocardial infarction, one due to COVID-19 (this patient was meant to receive baricitinib 4 mg but actually received baricitinib 2 mg), and one due to sepsis; there were three deaths in placebo treated patients, all due to respiratory failure.2
Conclusion
Despite positive results in a phase II trial in patients with SLE, baricitinib failed to meet the major secondary endpoints in both trials, with the primary endpoint not being met in SLE-BRAVE-II.1,2 However, there were no new safety signals observed in either trial.1,2
SLE-BRAVE-X, a phase III long-term extension in adults who completed SLE-BRAVE-II and -II, evaluated the safety and efficacy of baricitinib over three years; however, development of baricitinib for SLE has now been discontinued due to the poor efficacy outcomes seen in SLE-BRAVE-I and -II.3
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

