All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Dapirolizumab pegol efficacy by subgroup in patients with SLE: Post-hoc analyses of the phase IIb RISE study
Systemic lupus erythematosus (SLE) is a heterogenous autoimmune disorder that affects multiple organ systems.1 Given its pathophysiological implications, the CD40 ligand has long been an attractive therapeutic target in SLE. Dapirolizumab pegol (DZP), a polyethylene glycol-conjugated antigen-binding (Fab’) fragment, is a novel therapeutic agent under development for the treatment of SLE. DZP targets CD40L but lacks the functional Fc domain responsible for platelet activation and aggregation; therefore, it mitigates the risk of thromboembolic events commonly observed with other anti-CD40L antibodies.1
After its proven efficacy and safety in two phase I clinical trials (NCT01093911; NCT01764594) a phase IIb randomized, placebo-controlled trial (RISE; NCT02804763) was conducted to evaluate the dose-response, efficacy, and safety of DZP in patients with SLE.1 Included patients were adults with moderate-to-severe active SLE who were receiving stable corticosteroids, antimalarials, or immunosuppressants. Patients were randomized to receive placebo or intravenously infused DZP (6/24/45 mg/kg) along with standard-of-care (SOC) treatment every 4 weeks until Week 24.1
The primary objective was to identify a dose-response relationship across doses of DZP and placebo, based on the primary efficacy endpoint of Week 24 British Isles Lupus Assessment Group (BILAG)-based Composite Lupus Assessment (BICLA) responder rate.1
BICLA response was defined as:
- BILAG 2004 improvement without worsening, defined as
- BILAG A domain scores at baseline improved to B/C/D;
- all BILAG B domain scores at baseline improved to C/D;
- no new BILAG A domain scores; and
- ≤1 new BILAG B domain score compared with baseline.
- no worsening in Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) score compared with baseline;
- no worsening in physician’s global assessment (PGA) compared with baseline; and
- no increase in concomitant SLE medications compared with baseline.
At Week 24, BICLA response rates did not fit any of the pre-specified dose-response models with statistical significance (best-fitting model [Emax], p = 0.07]; therefore, the primary efficacy endpoint was not met (Figure 1). However, DZP had acceptable safety profile and demonstrated general improvements in other clinical and immunological measures of disease activity (BICLA, SLE Responder Index-4 [SRI-4], SLE Disease Activity Index 2000, PGA, and BILAG) after 24 weeks compared with placebo.1
Figure 1. BICLA response rate at Week 24 in patients with SLE*
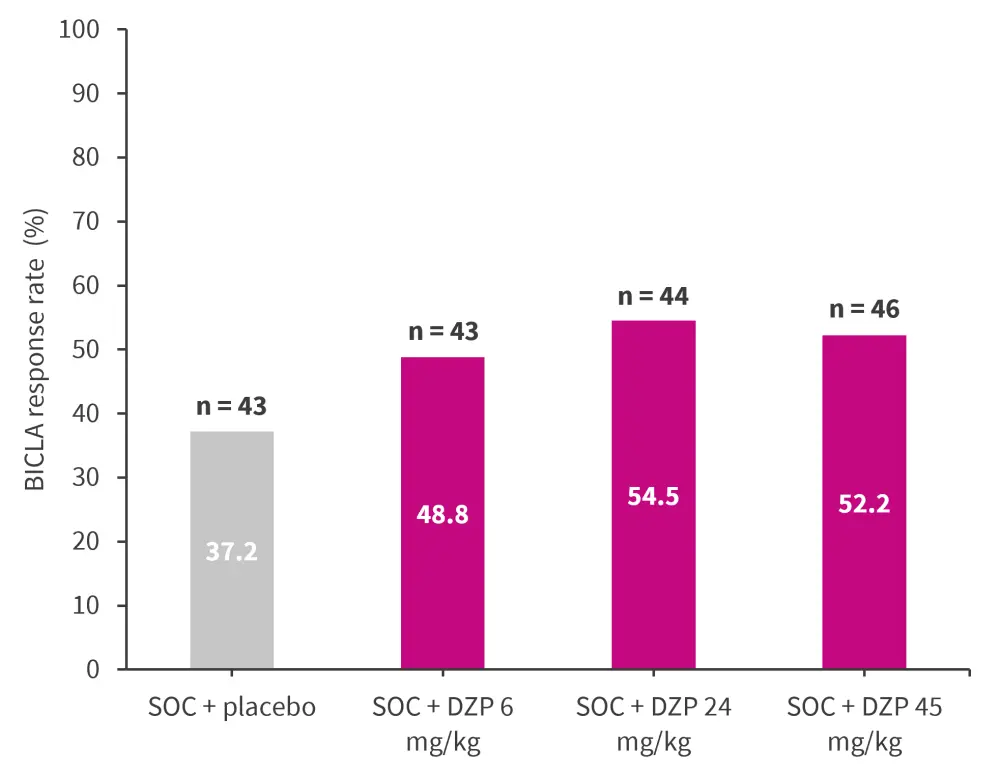
BICLA, British Isles Lupus Assessment Group-based Composite Lupus Assessment; DZP, dapirolizumab pegol; SLE, systemic lupus erythematosus; SOC, standard of care.
*Data from Furie RA, et al.1
Based on data from previous subgroup analyses of the phase IIb RISE trial, acute flare with normal complement levels was indicated as a potential predictor of high placebo response rates.2
At the European Alliance of Associations for Rheumatology (EULAR) 2023 congress, Askanase et al.2 presented post-hoc analyses assessing the treatment effects of DZP in subgroups from the phase IIb RISE trial who had characteristics identified as potential predictors of placebo response.2 Below, we summarize the key findings.
Methods2
In this post-hoc analysis, patients with SLE receiving placebo or DZP (6/24/45 mg/kg) alongside SOC were divided into subgroups based on previously identified potential predictors of placebo response, including
- disease activity at screening, defined using BILAG 2004 item scores either as acute flare (worsening/new symptoms) or persistent disease activity; and
- complement (C3/C4) levels at screening as either normal C3/C4 or low C3/C4 (C3, <0.0 g/L; C4, <180 mg/L).
The outcomes assessed were:
- BICLA response
- SRI-4 response
- PGA change from baseline
Results2
A total of 131 patients with acute flare + low C3/C4 or persistent disease activity and 47 patients with acute flare + normal C3/C4 were included in the analysis. Baseline patient characteristics of the combined subgroups are presented in Table 1.
Table 1. Baseline patient characteristics*
|
BILAG, British Isles Lupus Assessment Group; SLE, systemic lupus erythematosus; SLEDAI-2K, SLE Disease Activity Index-2000. |
||
|
Characteristics, % (unless otherwise stated) |
Acute flare with low C3/C4 or persistent disease activity |
Acute flare with normal C3/C4 (n = 47) |
|---|---|---|
|
Region |
|
|
|
Western/Central Europe |
17.8 |
8.5 |
|
Eastern Europe |
19.3 |
38.3 |
|
North America |
27.4 |
34.0 |
|
Latin America |
35.6 |
19.1 |
|
Mean time since first diagnosis of SLE, years |
8.2 |
8.1 |
|
≥1 organ system with BILAG Grade A |
40.7 |
57.4 |
|
≥2 organ systems with BILAG Grade B and no organ system with BILAG Grade A |
59.3 |
42.6 |
|
SLEDAI-2K Total Score <10 |
24.4 |
44.7 |
|
SLEDAI-2K Total Score ≥10 |
74.8 |
53.2 |
|
Patients taking systemic corticosteroids |
89.6 |
80.9 |
At Week 24, a higher proportion of patients receiving SOC + placebo with acute flare achieved BICLA response compared with those experiencing persistent disease activity (Figure 2A). A similar response was observed with patients with normal C3/C4 versus low C3/C4 (Figure 2B). In combined subgroups, a higher proportion of patients with acute flare + normal C3/C4 receiving SOC + placebo achieved BICLA response at Week 24, compared with patients with acute flare + low C3/C4 or persistent disease activity (Figure 2C).
Figure 2. BICLA response at Week 24 in patients receiving SOC + placebo for A disease activity, B complement levels, and C combined subgroups*
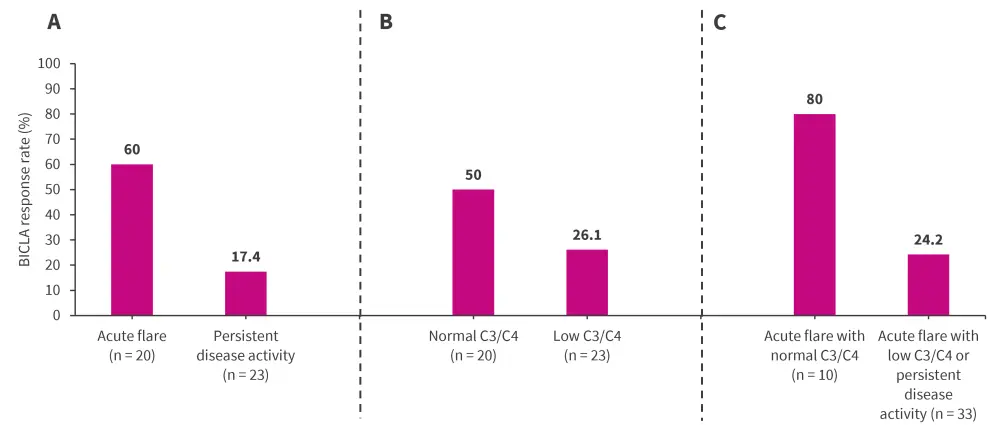
BICLA, British Isles Lupus Assessment Group-based Composite Lupus Assessment; SOC, standard of care.
*Data from Askanase, et al.1
Overall, between those receiving SOC + placebo versus SOC + DZP in the combined subgroups, higher BICLA response rates were achieved across all treatment arms in those patients with acute flare + normal C3/C4 levels when compared with those with acute flare + low C3/C4 levels or persistent disease activity. This pattern was more prominent in patients receiving SOC + placebo, with a significant 3-fold increase in BICLA response between subgroups (80.0% vs 24.2%; p = 0.005; Figure 3A).
Patients receiving SOC + placebo and SOC + DZP 6 mg/kg in the acute flare with normal C3/C4 subgroup achieved numerically higher SRI-4 response than patients in the acute flare with low C3/C4 or persistent disease activity subgroup. Responses were comparable in patients receiving SOC + DZP 24/45 mg across both the subgroups (Figure 3B).
At Week 24, a significantly greater change from baseline in PGA was achieved in patients receiving SOC + placebo in the acute flare with normal C3/C4 subgroup compared with the other subgroup; outcomes were comparable in patients receiving SOC + DZP (Figure 3C).
Figure 3. Efficacy outcomes by subgroup, including A BICLA response rate at Week 24, B SRI-4 response rate at Week 24, and C change from baseline in PGA at Week 24 in patients with SOC + placebo or DZP*
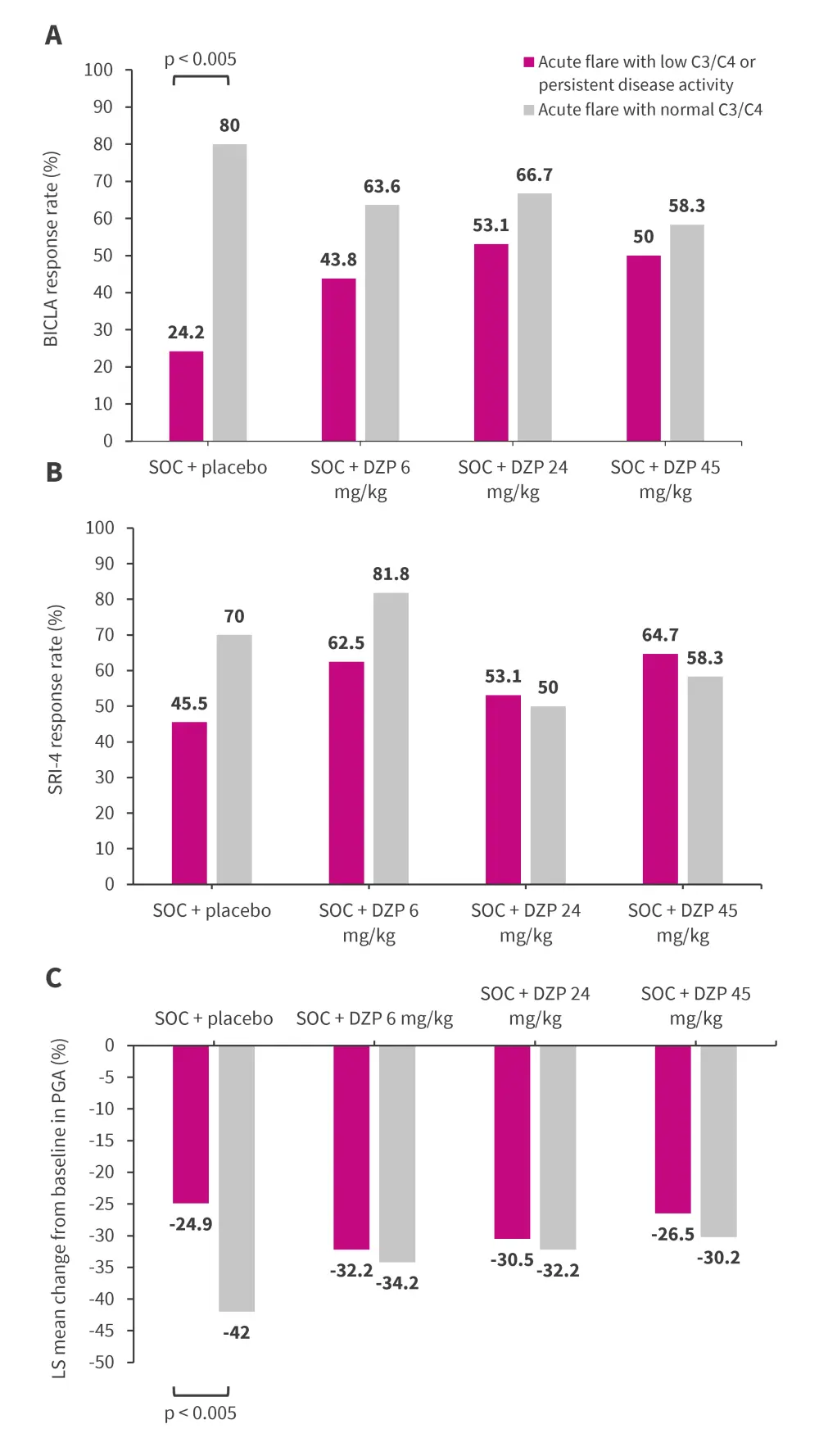
BICLA, British Isles Lupus Assessment Group-based Composite Lupus Assessment; DZP, dapirolizumab pegol; LS, least square; PGA, physician global assessment; SLE, systemic lupus erythematosus; SOC, standard of care; SRI-4, SLE Responder Index-4.
*Adapted from Askanase A, et al.2
Conclusion1
This post-hoc subgroup analysis showed that patients with acute flare and normal complement levels were more likely to achieve a response with SOC + placebo than patients with acute flare and low complement levels or persistent disease activity.
These findings highlight the importance of considering baseline clinical and serological activity patterns in SLE clinical trials to ensure adequate assessment of efficacy outcomes.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

