All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Efficacy and safety of anti-CD19 CAR T-cell therapy in patients with refractory systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is an autoimmune disease affecting multiple organs, characterized by an aberrant immune response, autoantibodies against nuclear antigens, such as double stranded deoxyribonucleic acid (dsNA), and organ inflammation.1
Currently, there is a lack of strategies to achieve drug-free remission or cure for SLE, with patients requiring lifelong treatment. Although treatments for SLE have substantially improved, some patients are refractory to these, leading to high risk of organ failure and mortality.1
B cells respond to DNA and nuclear antigen prior to the onset of SLE, making B-cell blockers an attractive therapeutic option.1 However, autoreactive B-cells are not accessible within lymphatic organs and inflamed tissues in SLE, posing challenges to the efficacy of B-cell depletion via CD20-targeting antibodies.1 Chimeric antigen receptor (CAR) T-cell therapy has emerged as a cancer treatment, with promising data in chronic lymphocytic leukemia, acute lymphoblastic leukemia, and B cell non-Hodgkin lymphoma leading to approvals of several CD19 CAR T-cell products1. Preclinical data from two studies2,3 support the efficacy of CD19 CAR T-cell therapy in SLE. Mackensen et al.1 recently published a study in Nature Medicine, evaluating the feasibility, efficacy, and tolerability of CD19 CAR T-cell therapy in patients with SLE. The key findings are summarized here.
Study design
Between February 2021 and February 2022, patients with a diagnosis of SLE according to the 2019 EULAR/ACR criteria, with signs of organ involvement, and who failed to respond to multiple immunomodulatory therapies were screened for the compassionate-use program. Figure 1 shows the treatment plan for patients with SLE receiving CD19 CAR T-cell therapy.
Figure 1. Treatment plan*
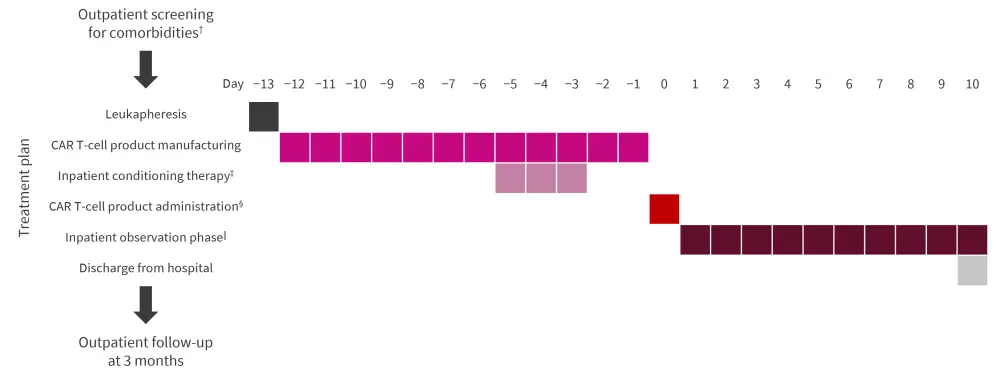
CAR, chimeric antigen receptor; IV, intravenously.
*Adapted from Mackensen, et al.1
†Patients were screened using chest X-ray, electrocardiography, echocardiography, and brain magnetic resonance imaging. Female patients also received a gynecologic consultation and administration of a gonadotropin-releasing hormone analogue to protect reproductive function prior to conditioning therapy.
‡Fludarabine 25 mg/m2/d (IV) from Day 5 to −3 and cyclophosphamide 1,000 mg/m2/d (IV) on Day −3.
§Administered as a single infusion (IV) on Day 0 at a fixed dose of 1 × 106 CAR T cells per kg body weight.
‖All patients remained hospitalized for daily toxicity monitoring for 10 days.
Transduction efficacy in vitro
Following leukapheresis, activated autologous T cells from patients were transduced using a lentiviral vector. These cells expanded >50‑fold and the final product showed a transduction efficacy of 20–40%.
B-cell elimination in vivo
After rapid expansion of the CAR T cells in all five patients, which peaked on average at Day 9 (11–59%), circulating CAR T-cell counts declined rapidly. From the second day after CAR T cell administration, B cells completely disappeared from patients’ peripheral blood. In comparison, CD4+/CD8+ T cells, monocytes, and neutrophils only temporarily decreased in number.
Results
Baseline characteristics
A total of five patients were included, of which four patients were female and one patient was male. All patients had a median age of 22 years (range, 18–24 years), active SLE, multiple organ involvement, and a Systemic Lupus Erythematosus Disease Activity Index-2000 (SLEDAI-2K) score between 8 and 16 (Table 1).
Table 1. Baseline characteristics*
|
C3, complement factor 3; dsDNA, double stranded DNA; HEM, hematological abnormalities; LEF, leflunomide; MTX, methotrexate; MYO, myositis; N, no; SER, serositis; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index-2000; TAC, tacrolimus; Y, yes. |
|||||
|
Characteristic |
Patient |
||||
|---|---|---|---|---|---|
|
1 |
2 |
3 |
4 |
5 |
|
|
Age, years |
22 |
23 |
22 |
24 |
18 |
|
Disease duration, years |
4 |
1 |
6 |
9 |
3 |
|
SLEDAI-2K score |
16 |
16 |
10 |
8 |
9 |
|
Laboratory parameters |
|||||
|
Baseline C3, mg/dL |
49 |
43 |
56 |
88 |
68 |
|
Baseline anti-dsDNA, U/mL |
5,600 |
2,060 |
479 |
4 |
52 |
|
Proteinuria, mg/24 hour |
2,015 |
3,080 |
6,539 |
8,096 |
88 |
|
Organ involvement, Y/N |
|||||
|
Skin |
Y |
Y |
Y |
Y |
Y |
|
Kidney (Stage) |
Y (III) |
Y (III) |
Y (IV) |
Y (III/V) |
Y (III/V) |
|
Joints |
N |
Y |
Y |
Y |
Y |
|
Lungs |
Y |
N |
Y |
Y/N |
N |
|
Heart |
Y |
N |
N |
Y |
N |
|
Other |
HEM |
N |
SER |
MYO |
HEM |
|
Previous treatment exposure, Y/N |
|||||
|
Glucocorticoid pluses |
Y |
Y |
Y |
Y |
Y |
|
Hydroxychloroquine |
Y |
Y |
Y |
Y |
Y |
|
Mycophenolate mofetil |
Y |
Y |
Y |
Y |
Y |
|
Azathioprine |
N |
N |
N |
Y |
Y |
|
Cyclophosphamide |
Y |
Y |
Y |
N |
N |
|
Rituximab |
Y |
N |
N |
N |
N |
|
Belimumab |
Y |
Y |
Y |
Y |
Y |
|
Others |
TAC |
N |
N |
MTX, LEF |
N |
Clinical efficacy
Disease activity assessment using SLEDAI-2K following administration of CD19 CAR T cells showed continuous improvement in all five patients. At 3 months after administration of CD19 CAR T cells, the SLEDAI-2K score was 0 in four patients. One patient (patient no. 2) showed a SLEDAI-2K score of 2 (Figure 2) along with residual low-level proteinuria after 3 months, which improved with dose adaptation of angiotensin-converting enzyme inhibitors.
All five patients met the definitions of DORIS remission criteria and Lupus Low Disease Activity State 3 months after treatment. Notably, in all five patients, immunomodulatory and immunosuppressive drugs could be discontinued, achieving drug-free remission.
In addition, nephritis ceased after CD19 CAR T-cell treatment, there was normalization of complement factor levels, and a reduction in anti-dsDNA antibody levels in all five patients. With regards to severe manifestations of SLE, the following either reduced or disappeared after administration of CAR T cells:
- Fatigue (all patients; numeric rating scale-based intensity reduced from 8.2 ± 1.4 at baseline to 2.4 ± 1.6 at 3 months; p = 0.007)
- Interferon alpha in serum (detectable in three patients at baseline and undetectable in all patients at follow-up)
- Fibrosis of cardiac valves (patient 1)
- Restriction and diffusion impairment of lungs (patients 1 and 3)
- Arthritis (patient 4)
Figure 2. Efficacy of CD19 CAR T cells on disease activity*
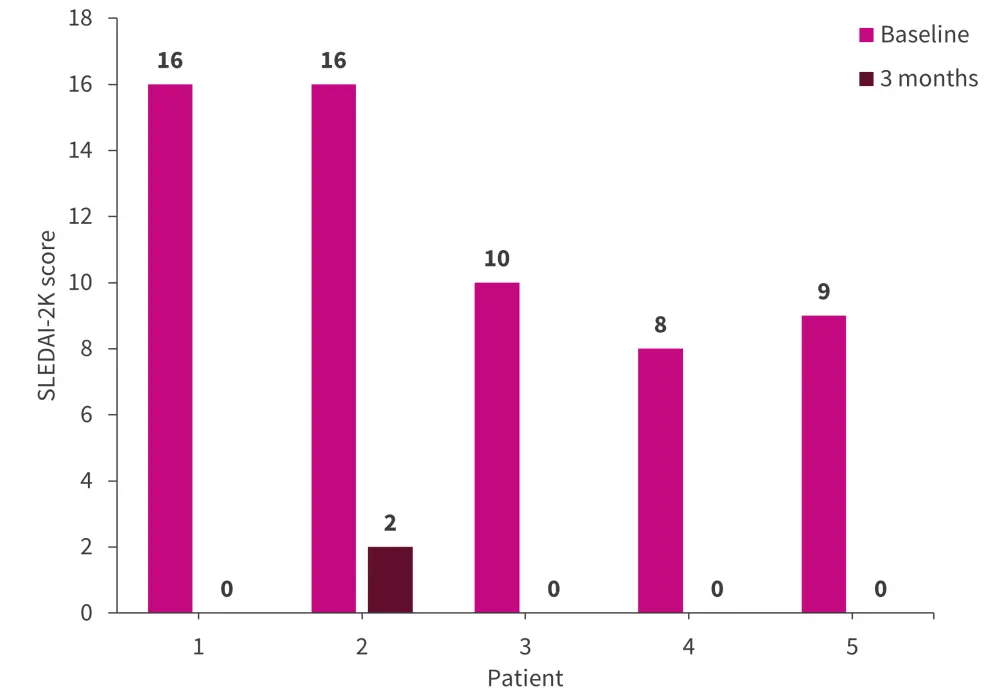
SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index-2000.
*Adapted from Mackensen et al.1
Immune and long-term effects
The anti-dsDNA antibodies disappeared at 3 months, along with a decrease in antibodies against nucleosomes, secondary necrotic cells, single-stranded DNA, Smith antigen, and Ro60. No antibodies against histones, Ro52, and SS-B/La were identified in any patients.
The follow-up time was 17, 12, 8, 7, and 5 months for patient 1, 2, 3, 4, and 5, respectively. The median time of B-cell reconstitution was 110 days (range, 63–142 days), with no evidence of SLE relapse observed in any patients during the long-term follow-up.
- Patient 1: B-cell reconstitution over 12 months
- Patient 2: Immune competent over 6 months
- Patient 3, 4, and 5: Immune reconstitution ranged from 1 to 3 months
Comparison of immune phenotypes of B cells before and after treatment showed that B cells were mostly CD21+CD27−-naïve cells, with very low levels or no detection of CD21+CD27+, memory B cells and CD38+CD20− plasmablasts. Also, CD11c+CD21lo memory B cells, which are known to be expanded in SLE, were not evident amongst recurring B cells. In addition, the authors noted a shift from class-switched immunoglobulin (Ig)G and IgA heavy chains at baseline to non-class-switched IgM and IgD heavy chains in the regenerated B cells.
Effects of vaccination antibody levels
Vaccination responses against measles, rubella, mumps, varicella zoster virus, and hepatitis B did not show a substantial decline, suggesting CAR T-cell therapy targeted autoantibody-producing cells rather than all immunoglobulin-producing cells.
Safety and tolerability
All five patients had no or only mild cytokine release syndrome (CRS), including CRS Grade 1 in three patients, which was successfully treated with metamizole. Four patients showed increased levels of C-reactive protein and interleukin-6 between Day 1 and 5. No immune effector cell-associated neurotoxicity syndrome or infections were observed.
Conclusion
Mackensen et al.1 conclude that the data from their study show CD19 CAR T-cell therapy is effective against depletion of B cells and can lead to drug-free remission in patients with SLE, and that the production and administration of CAR T cells in patients with autoimmune disease is feasible and tolerable. The results show a rapid and continual breakdown of B cell-mediated autoimmune response after CD19 CAR T-cell therapy, indicating the abrogation of underlying autoimmune processes of SLE. In addition, the authors did not report any flare of SLE despite B-cell reconstitution 100 days following CAR T-cell therapy. The immune effects and anti-vaccination responses remained stable, suggesting that most long-lived plasma cells are not targeted by CAR T cells. Although the results from this case series are promising with this potential new treatment option, the data are limited by a small sample size and uncertainty around the influence of specific patient characteristics and conditioning regimens on treatment responses. Therefore, trials of larger cohorts with longer follow-up are needed to validate the continued absence of autoimmunity and resolution of inflammation in patients with SLE treated with CAR T-cell therapy.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

