All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Efficacy and safety of RSLV-132 in patients with SLE: A phase IIa trial
Circulating, extracellular ribonucleic acid is a potent activator of type I interferon which plays a central pathogenic role in systemic lupus erythematosus (SLE). RSLV-132, a catalytically active human RNase I fused with human immunoglobulin G1 fragment crystallizable region, is designed to degrade extracellular RNA, and alleviate chronic inflammation linked with SLE.
Here, we summarize an article by Burge et al.1 published in Lupus Science & Medicine, evaluating the efficacy and safety of RSLV-132 in patients with SLE in a phase IIa trial.
Study design1
- This was a double-blind, placebo-controlled, phase IIa, proof-of-concept study in patients with SLE with moderate-to-severe cutaneous disease activity.
- Patients were randomized 2:1 to receive 13 doses (6 months of weekly loading dose followed by 5 months of biweekly dose) of 10 mg/kg RSLV-132 or placebo.
- The primary endpoint was a change in Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) score from baseline to Day 169.
- Secondary endpoints included change from baseline in Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K), the British Isles Lupus Assessment Group 2004 Index (BILAG-2004), and the Physician’s Global Assessment (PGA).
- A subgroup of participants with baseline CLASI score ≥21 and SLEDAI score ≥9 were analyzed with respect to British Isles Lupus Assessment Group-based Composite Lupus Assessment (BICLA) and SLE Responder Index (SRI)-4 responses.
Key findings1
- Out of the 65 patients who met the eligibility criteria, 12 in the placebo group (mean age, 45.2 years; female, 100%) and 25 in the RSLV-132 group (mean age, 45.3 years; female, 93%) completed the study.
Safety
- The incidence of treatment-emergent adverse events (AE), treatment-related AE, treatment discontinuation, and study termination due to treatment-emergent AE were comparable between arms, while the incidence of treatment-emergent serious adverse events was lower in the RSLV-132 group compared with the placebo group (7.1% vs 22.7%).
- No deaths were reported
- No participants were positive for anti-RSLV-132 antibodies.
Efficacy
- There were no significant differences in the mean change in CLASI score, SLEDAI-2K, PGA, and BILAG improvement between the placebo and RSLV-132 groups (Figure 1).
Figure 1. Change from baseline in A CLASI, SLEDAI-2K, PGA, and B BILAG*
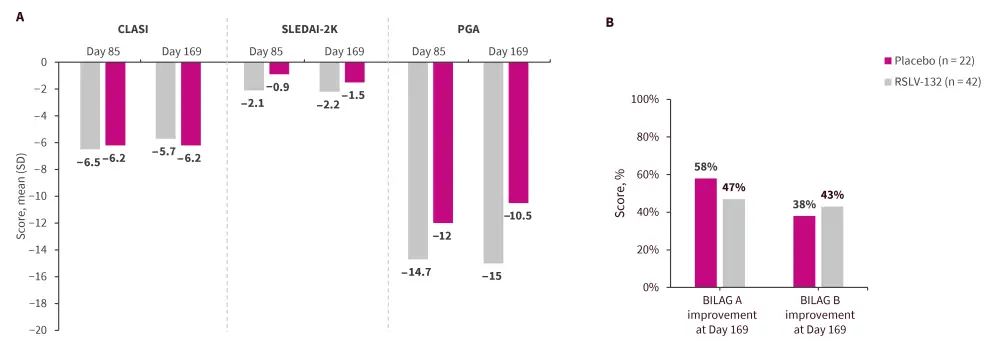
BILAG, British Isles Lupus Assessment Group; CLASI, Cutaneous Lupus Erythematosus Disease Area and Severity Index; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000.
*Data from Burge, et al.1
- In patients with higher SLEDAI score (placebo, n = 9; RSLV-132, n = 13) and CLASI score (placebo, n = 12; RSLV-132, n = 18), the RSLV-132 treated group exhibited increased BICLA, SRI-4, and CLASI-50 responses compared with the placebo group (Figure 2).
Figure 2. Composite analysis endpoint*
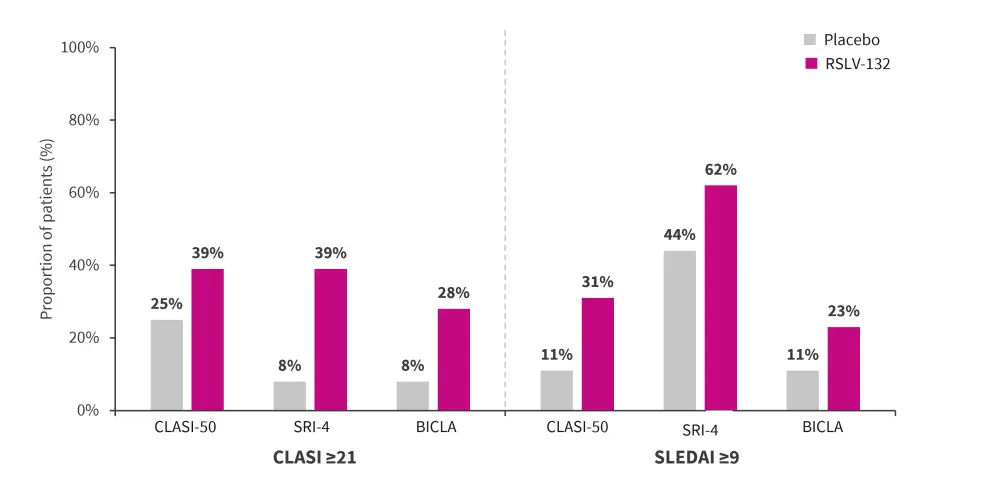
BICLA, British Isles Lupus Assessment Group-based Composite Lupus Assessment; CLASI, Cutaneous Lupus Erythematosus Disease Area and Severity Index; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000; SRI, SLE Responder Index 4.
*Adapted from Burge, et al.1
|
Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

