All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Efficacy and safety of telitacicept in patients with SLE: Results from a phase III trial
Telitacicept, a recombinant fusion protein consisting of the extracellular domain of the transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) receptor + the fragment crystallizable region (Fc) domain of human immunoglobulin G (IgG), is a novel therapeutic agent for systemic lupus erythematosus (SLE).1 The extracellular domain of the TACI receptor binds and suppresses B-lymphocyte stimulator (BLyS) and proliferation-inducing ligand (APRIL) signaling, while the Fc domain of human IgG increases the stability of telitacicept.1
Blocking BLyS can prevent the maturation of immature B cells and therefore prevent the recurrence of the disease.2 Blocking APRIL can inhibit the differentiation of mature B cells into plasma cells and thereby prevent the release of autoantibodies by autoreactive plasma cells, which can effectively suppress immune responses and manage the autoimmune symptoms.2
At the European Alliance of Associations for Rheumatology (EULAR) 2023 Congress, Van Vollenhoven et al. presented phase III trial results on the efficacy and safety of telitacicept in patients with SLE.1 Below, we summarize the key findings.
Methods1
This was a double-blind, randomized, placebo-controlled phase III study assessing the efficacy and safety of telitacicept in patients with SLE. The study design is illustrated in Figure 1.
Figure 1. Study design*
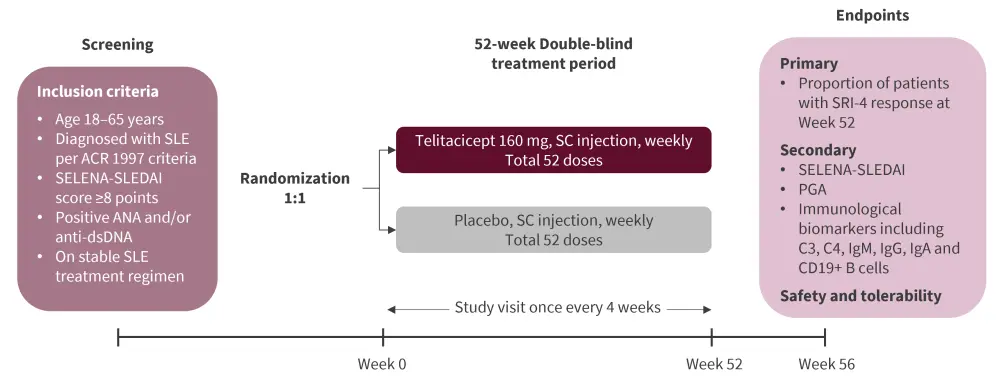
ACR, American College of Rheumatology; ANA, antinuclear antibody; Anti-dsDNA, anti-double-stranded deoxyribonucleic acid antibody; Ig, immunoglobulin; PGA, Physician’s Global Assessment; SC, subcutaneous; SELENA-SLEDAI, Safety of Estrogens in Lupus National Assessment-Systemic Lupus Erythematosus Disease Activity Index; SLE, systemic lupus erythematosus; SRI, SLE responder index.
*Adapted from Van Vollenhoven et al.1
Results1
A total of 335 patients with SLE were included, of which 167 received telitacicept 160 mg and 168 received placebo. Both groups had comparable baseline demographics and disease characteristics. Mean age was ≈35 years and >90% patients were female (Table 1).
Table 1. Baseline demographics and disease characteristics*
|
ANA, antinuclear antibody; dsDNA, double-stranded DNA; BILAG, British Isles Lupus Assessment Group; BMI, body mass index; PGA, physician’s global assessment; SD, standard deviation; SLE, systemic lupus erythematosus; SELENA-SLEDAI, Safety of Estrogens in Lupus National Assessment-Systemic Lupus Erythematosus Disease Activity Index. |
||
|
Characteristics, % (unless otherwise stated) |
Placebo |
Telitacicept 160 mg |
|---|---|---|
|
Female |
93.5 |
91.0 |
|
Mean age (SD), years |
35.1 (10.45) |
34.8 (9.77) |
|
Mean BMI (SD), kg/m2 |
22.67 (3.89) |
23.01 (3.41) |
|
Mean SLE disease duration (SD); years |
7.21 (5.32) |
7.54 (5.53) |
|
Mean SELENA-SLEDAI score (SD) |
11.5 (3.64) |
11.5 (3.14) |
|
At least BILAG 1A or 2B |
59.5 |
62.9 |
|
Mean PGA (SD) |
0.47 |
0.44 |
|
Serum biomarkers |
||
|
Low C3 and/or C4 |
70.2 |
71.3 |
|
Positive ANA |
98.2 |
95.8 |
|
Mean proteinuria (SD) |
1.237 (1.343) |
1.395 (1.571) |
|
Glucocorticoid (prednisone equivalent) dose >7.5 mg/day |
73.8 |
70.7 |
|
Monotherapy |
||
|
Glucocorticoid |
0 |
0.6 |
|
Antimalarials |
1.8 |
0 |
|
Immunosuppressive drugs |
0.6 |
0.6 |
|
Combination therapy |
||
|
Glucocorticoids + antimalarials |
15.5 |
15.0 |
|
Glucocorticoids + immunosuppressives |
14.3 |
13.8 |
|
Glucocorticoids + immunosuppressives + antimalarials |
67.9 |
70.1 |
Efficacy1
The study met its primary endpoint as a significantly greater proportion of patients receiving telitacicept achieved SRI-4 response at Week 52 compared with those receiving placebo (Figure 1). Additionally, SRI-4 response was sustained for up to Week 52 in the telitacicept group. Time to first SLE flare was significantly longer among patients receiving telitacicept compared with placebo (median 95% confidence interval [CI], 198 [169–254] days vs 115 [92–140] days, respectively; p < 0.001).
Figure 2. SRI-4 response rate at Week 52 (FAS mITT)*†
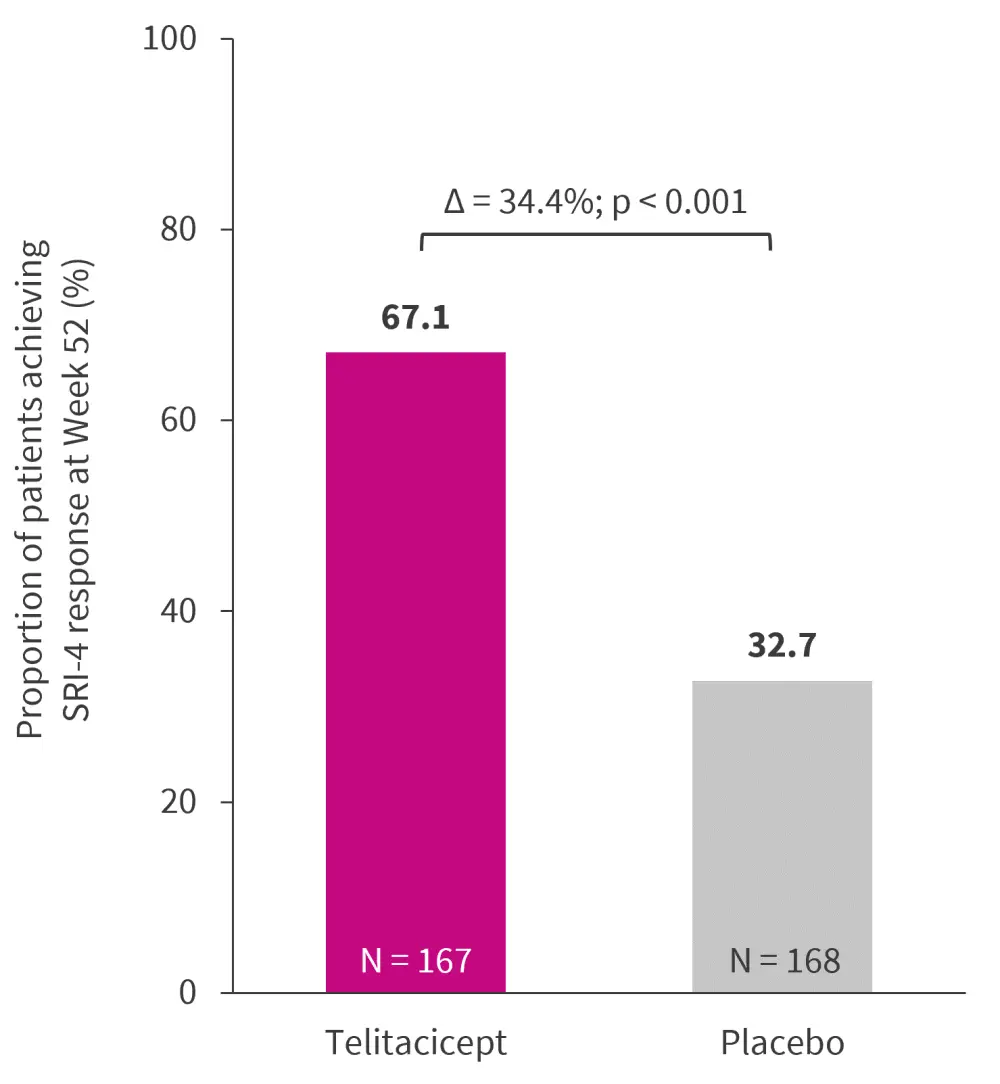
FAS, full analysis set; mITT; modified intention-to-treat; SRI, SLE responder index.
*Adapted from Van Vollenhoven et al.1
†Missing data imputed as ‘non-response’.
The key secondary outcomes are depicted in Figure 2. A significantly greater proportion of patients treated with telitacicept showed improvement in SELENA-SLEDAI and PGA compared with those treated with placebo (p < 0.001).
Figure 3. Key secondary endpoints*
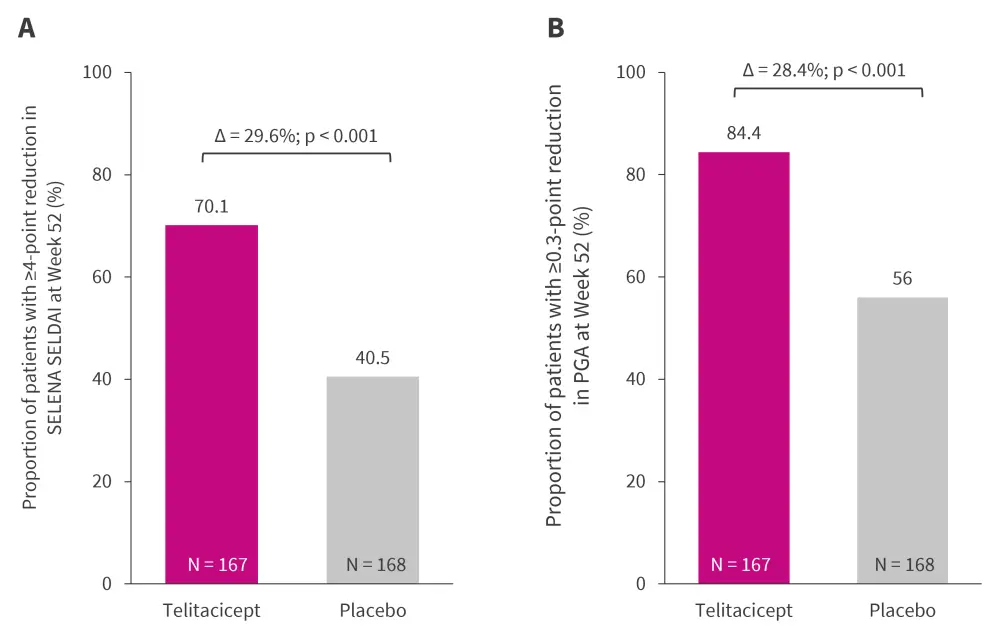
PGA, physician’s global assessment; SELENA-SLEDAI, Safety of Estrogens in Lupus National Assessment-Systemic Lupus Erythematosus Disease Activity Index.
*Data from Van Vollenhoven, et al.1
Additionally, telitacicept treatment was associated with a rapid and sustained increase of complement levels (C3 and C4) and reduction of immunoglobulins (IgM, IgG, and IgA) and CD19+ B cells.
Safety and tolerability1
Most treatment-emergent adverse events (TEAEs) were of mild to moderate severity (Table 2). TEAEs and infections were comparable between the treatment groups, while the incidence of serious AEs and serious infections was higher in the placebo versus telitacicept group. There were no deaths reported in either group.
Table 2. Safety and tolerability*
|
SAE, serious adverse event; SOC, system organ class; TEAE, treatment-emergent adverse event. |
||
|
Event, % (unless otherwise stated) |
Placebo |
Telitacicept |
|---|---|---|
|
TEAEs |
84.5 |
91.6 |
|
Drug-related TEAEs |
50 |
74.9 |
|
TEAE leading to discontinuation |
5.4 |
4.8 |
|
SAEs |
2.4 |
1.2 |
|
Infections and infestations (SOC)† |
60.1 |
65.3 |
|
Upper respiratory tract infections |
32.7 |
42.5 |
|
Urinary tract infection |
15.5 |
11.4 |
|
Nasopharyngitis |
6.5 |
2.4 |
|
Herpes zoster |
3.6 |
4.8 |
|
Gastroenteritis |
3.0 |
5.4 |
|
Serious infections |
5.4 |
1.2 |
|
Severe infections |
4.8 |
1.2 |
|
Psychiatric disorders |
3.0 |
2.4 |
Conclusion1
This phase III trial of telitacicept in patients with SLE met its primary efficacy endpoint. Treatment with telitacicept (160 mg weekly) demonstrated favorable clinical outcomes and an acceptable safety profile in patients with moderate to severe SLE.
Telitacicept obtained its first approval in China in 2021 for the treatment of patients with active SLE. Currently, a global phase III trial (REMESLE-1) is at the enrolment stage.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

