All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
How might deucravacitinib fit into the treatment pathway for patients with SLE?
Featured:
During the Lupus Hub Steering Committee meeting, key opinion leaders discussed how deucravacitinib might fit into the treatment pathway for patients with systemic lupus erythematosus (SLE). This recorded discussion was led by committee chair Ricard Cervera, and featured Murray Urowitz, Mandana Nikpour, Betty Diamond, James Cheng-Chung Wei, Manuel Francisco Ugarte-Gil, and Lyndsy Ambler.
How might deucravacitinib fit into the treatment pathway for patients with SLE?
How might deucravacitinib fit into the treatment pathway for patients with SLE?
Following the recently published phase II trial data of deucravacitinib for the management of SLE,1 and the U.S. Food and Drug Administration (FDA) approval of this drug for psoriasis,2 the committee members discuss how this TYK2 inhibitor might fit into the current treatment algorithm for patients with SLE.
Current treatment pathways
Although deucravacitinib is not currently approved for use in the treatment of lupus, phase II testing has been completed and phase III trials are ongoing (POETYK SLE‑1 [NCT05617677] and POETYK SLE‑2 [NCT05620407]). Current treatments for lupus are outlined in Figure 1.
Figure 1. Lupus treatment overview*
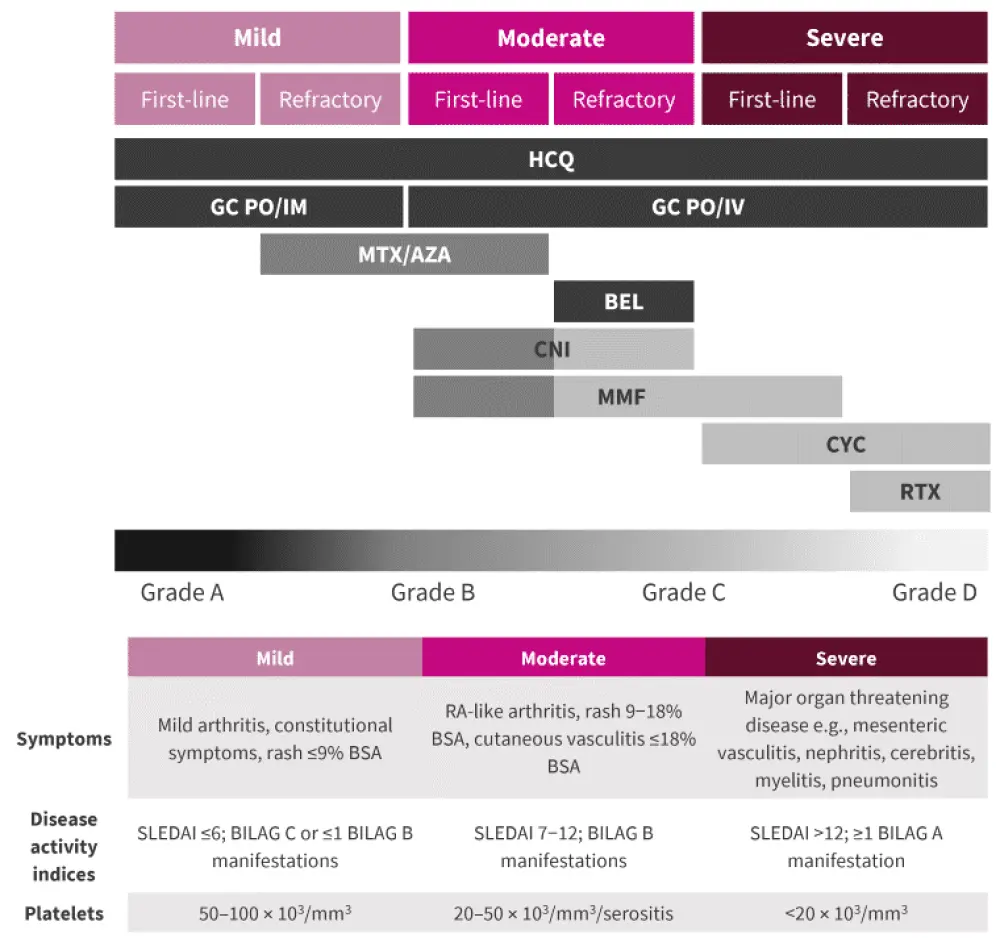
BEL, belimumab; BILAG, British Isles Lupus Assessment Group Disease Activity Index; BSA, body surface area; CNI, calcineurin inhibitor; CYC, cyclophosphamide; GC, glucocorticoids; HCQ, hydroxychloroquine; IM, intramuscular; IV, intravenous; MMF, mycophenolate mofetil; MTX, methotrexate; PO, per os; RA, rheumatoid arthritis; RTX, rituximab; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index.
*Adapted from Fanouriakis, et al.1
Case study
This discussion is facilitated in the form of a case study to consider a patient with moderate-to-severe SLE; already receiving glucocorticoids plus immunosuppressant and antimalarials, but inadequately controlled.
Possible next steps for the treatment of this patient include:
- Increasing immunosuppressant dose if suboptimal
- Change the immunosuppressant
- Introducing immune cell-targeted therapies, such as belimumab or rituximab
Unmet needs and future therapies
Most current treatment pathways require a therapy to fail before the introduction of an alternative. However, there is debate on whether this treatment approach may be improved by the earlier introduction of alternatives or combination therapies.
Deucravacitinib is an oral treatment that may be less immunosuppressive than standard-of-care therapies, which could reduce the number of infections patients experience. The phase II trial of deucravacitinib in SLE has also been one of the first to mandate steroid reduction, which could help to differentiate the agent from the placebo. The phase II study reported higher rates of rashes and acne in patients receiving deucravacitinib, which the committee felt might be difficult for patients with SLE, unless it was found to be more efficacious than current options, such as belimumab.
Combination therapies
Lupus possesses a multifactorial pathogenesis, suggesting that a combination therapy approach may be effective to treat multiple different aspects of the disease. The committee was excited by the prospect of combination treatment utilizing different mechanisms of action, such as belimumab plus deucravacitinib, which may be effective for symptom reduction and earlier cessation.
Conclusion
Data available thus far on deucravacitinib as a therapy for SLE are promising; however, no conclusions can be made until more data from phase III trials are available. There are indications that deucravacitinib may fit into the current treatment algorithm as an alternative to failed therapies or potentially in combination for a multi-targeted approach.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Betty Diamond
Betty Diamond Murray Urowitz
Murray Urowitz Ricard Cervera
Ricard Cervera Manuel Francisco Ugarte-Gil
Manuel Francisco Ugarte-Gil Mandana Nikpour
Mandana Nikpour James Cheng-Chung Wei
James Cheng-Chung Wei