All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Long-term voclosporin use in lupus nephritis: Results from AURORA 2
At the American College of Rheumatology (ACR) Convergence 2022, Arriens1 presented results from the phase III AURORA 2 continuation study. The double-blind AURORA studies investigated the use of voclosporin in patients with lupus nephritis (LN) already receiving treatment with mycophenolate mofetil and/or oral steroids, compared with control.1
LN is a frequent complication in patients with systemic lupus erythematosus and LN episodes are associated with kidney failure.1 Voclosporin is a calcineurin inhibitor that was approved in 2021 by the U.S. Food and Drug Administration (FDA) for use in adults with active LN.2 Results from the phase III AURORA 1 study showed that patients receiving voclosporin had significantly increased complete renal response rates compared with the control group (briefly summarized in our previous article on the management of LN here).1
Study design1
In AURORA 1, patients followed a rapid glucocorticoid taper schedule (Figure 1). The final dose of glucocorticoid in AURORA 1 was the starting dose in AURORA 2, which could then be adjusted further by the study investigator. AURORA 2 was a continuation study evaluating 216 patients for an additional 2 years. Both studies were blinded, placebo-controlled studies.
Figure 1. Study design of AURORA 1 and AURORA 2 and the glucocorticoid tapering schedule in AURORA 1*
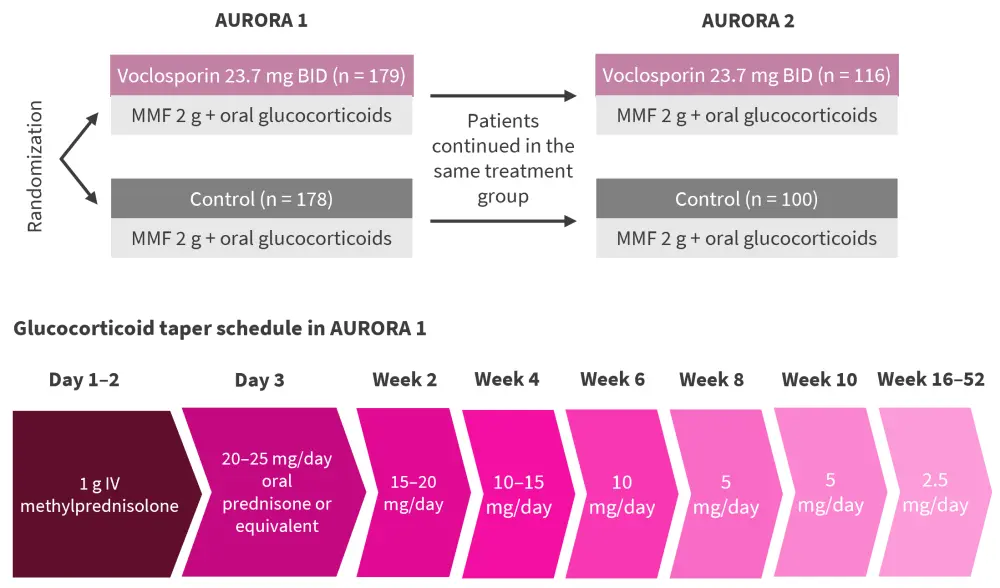
BID, twice daily; IV, intravenous; MMF, mycophenolate mofetil.
*Adapted from Arriens.1
Results1
In AURORA 2, patient characteristics at baseline were generally well balanced between arms (Table 1).
Table 1. Baseline patient characteristics in AURORA 2*
|
eGFR, estimated glomerular filtration rate; LN, lupus nephritis; UPCR, urine protein creatinine ratio. |
||
|
Characteristic |
Control |
Voclosporin |
|---|---|---|
|
Mean age, years |
35.4 |
32.3 |
|
Female, % |
88.0 |
90.5 |
|
Race, % |
|
|
|
White |
40.0 |
37.9 |
|
Asian |
30.0 |
25.9 |
|
Black |
7.0 |
15.5 |
|
Other |
23.0 |
20.7 |
|
Mean time since initial LN diagnosis, years |
4.8 |
5.0 |
|
Pretreatment corrected eGFR, mL/min/1.73 m2 |
78.9 |
79.6 |
|
Pretreatment UPCR, mg/mg |
3.9 |
3.9 |
Safety
The rates of adverse events in both groups decreased over the 3 years, with a higher percentage of renal adverse events in patients treated with voclosporin (Table 2). Estimated glomerular filtration rate reductions and hypertension occurred at a higher rate in Year 1 in voclosporin-treated patients compared with the control group (19% vs 6%, respectively, and 20.7% vs 6%, respectively), but rates of these events decreased over time (at Year 3; 3.9% vs 2.4%, respectively, and 2.9% vs 2.4%, respectively). The mean change in estimated glomerular filtration rate from the baseline of AURORA 2 (Month 12 following the start of AURORA 1) to Month 36 was minimal in both voclosporin and control patients.
Table 2. Overall adverse events during AURORA 1 and 2*
|
eGFR, estimated glomerular filtration rate. |
||
|
Adverse event, % |
Control |
Voclosporin |
|---|---|---|
|
Adverse event |
95.0 |
92.2 |
|
Serious adverse event |
28.0 |
26.7 |
|
Treatment-related serious adverse event |
4.0 |
4.3 |
|
eGFR decreased |
9.0 |
24.1 |
|
Hypertension |
13.0 |
26.7 |
|
Renal impairment |
2.0 |
5.2 |
|
Acute kidney injury |
0 |
2.6 |
Efficacy
The reductions in urine protein creatinine ratio (UPCR) seen in AURORA 1 were maintained until the 4-week follow-up following study drug discontinuation of AURORA 2. More patients treated with voclosporin achieved a UPCR ≤0.5 mg/mg compared with the control at all time points (Figure 2).
Figure 2. Proportion of patients achieving a UPCR ≤0.5 mg/mg*
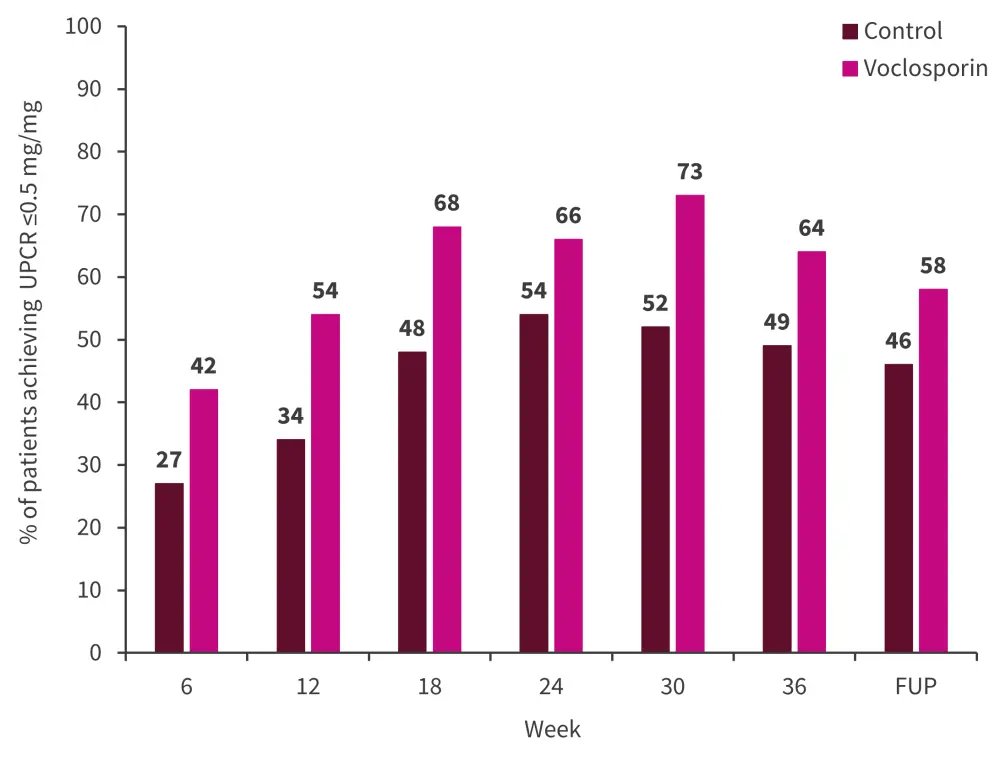
FUP, follow-up; UPCR, urine protein creatinine ratio.
*Adapted from Arriens.1
A good renal outcome, defined as an adequate response (sustained reduction in UPCR ≤0.7 mg/mg) without renal flare (an increase in UPCR to >1.0 mg/mg from post-response baseline <0.2 mg/mg or >2 mg/mg from post-response baseline of 0.2–1.0 mg/mg), was achieved by significantly more patients receiving voclosporin (66.4%) compared with control (54%; odds ratio, 0.56; 95% confidence interval, 0.32–0.99; p = 0.045).
Conclusion
The reductions in UPCR seen in patients treated with voclosporin in AURORA 1 were maintained until the end of AURORA 2 and into the follow-up period. A total of 66.4% of patients treated with voclosporin achieved a good renal response, with no new safety signals. In addition, although calcineurin inhibitors, such as voclosporin, can cause kidney injury and thus cause a decrease in eGFR, the initial decrease in eGFR recovered to baseline levels and remained stable through the 3 years in voclosporin-treated patients and following the completion of study drug treatment. Arriens1 concluded that AURORA 2 demonstrates voclosporin can be used safely long term in combination with mycophenolate mofetil and steroids for patients with LN.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


