All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Editorial theme | Outcome measures in SLE: the current landscape and future perspectives
Do you know... Which outcome measure used in lupus clinical trials can be defined as: measurement of overall disease activity often using a scale of ≤1 (mild), 1–2 (moderate), and 2–3 (severe)?
In recent years, many promising treatments for systemic lupus erythematosus (SLE) have failed to meet the primary efficacy endpoint in phase III trials, with the exception belimumab in the BLISS-52 and BLISS-76 trials (NCT00424476; NCT00410384).1 It has been suggested that current lupus clinical trial outcome measures do not adequately detect improvement and worsening in symptoms and disease activity.1
A commonly used endpoint in SLE trials is the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), a global disease activity score that may not reflect simultaneous improvement or worsening in disease severity across different organs. Another endpoint, frequently used for organ assessment, is the British Isles Lupus Assessment Group (BILAG); this measure may not have the ability to differentiate between multiple events within one organ.
Due to these difficulties, composite measures have been created which are able to detect improvement (without worsening) in SLE disease activity. These measures are the SLE Responder Index (SRI) and the BILAG-Based Composite Lupus Assessment (BICLA). Here, we discuss the different outcome measures currently used in SLE clinical trials, the results from a study comparing SRI and BICLA against a physician’s judgement of reponse,1 and provide an overview of new outcome measures in development.
Outcome measure definitions2
Figure 1 summarizes the current outcome measures used in SLE clinical trials.
Figure 1. Definitions of the current outcome measures used in SLE trials*
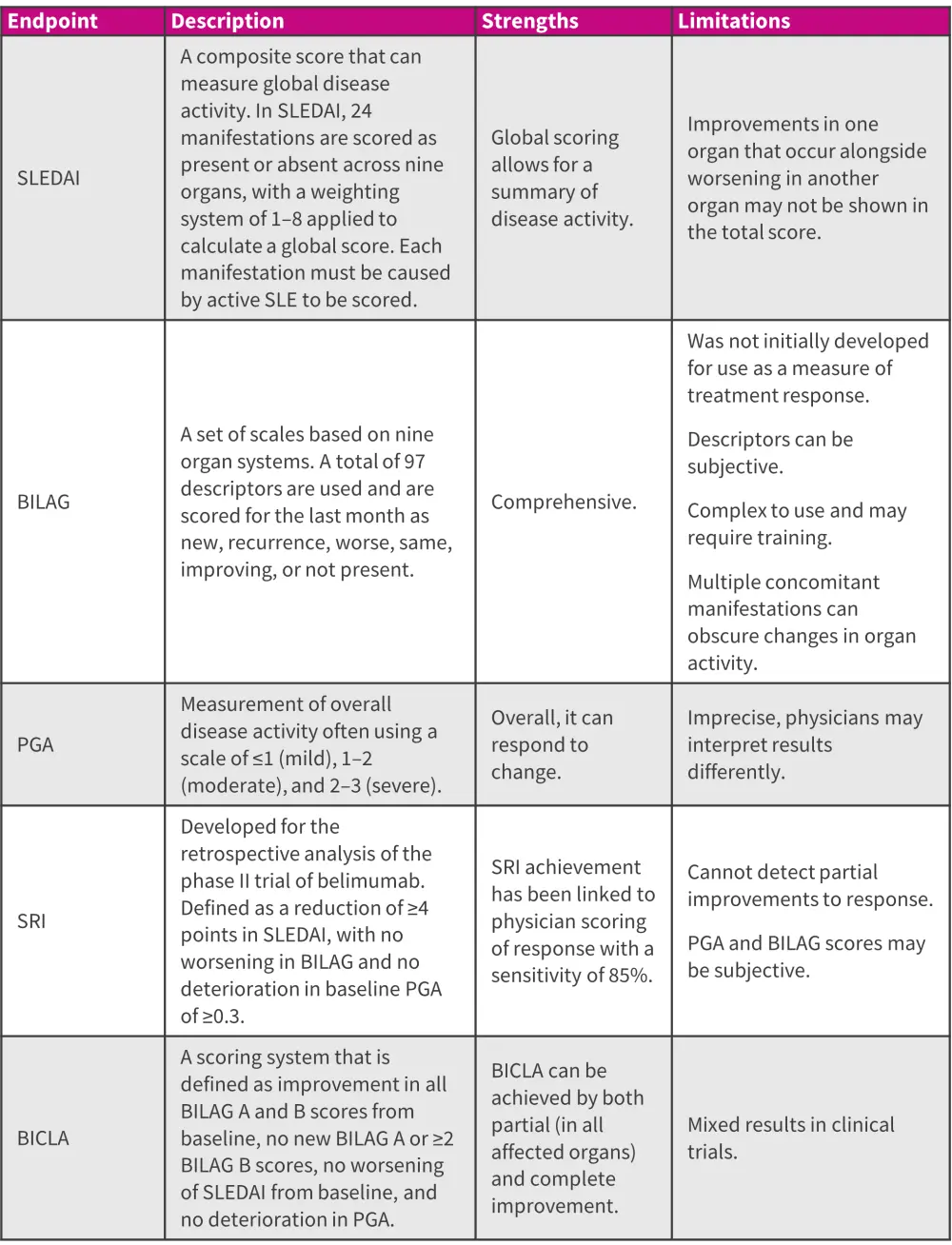
BICLA, BILAG-Based Composite Lupus Assessment; BILAG, British Isles Lupus Assessment Group; PGA, physician’s global assessment; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; SRI, Systemic Lupus Erythematosus Responder Index.
*Data from Connelly, et al.2
SRI and BICLA compared to PRI1
Study design
Patients from the Oklahoma Lupus Cohort study were enrolled if they had SLE according to the 1997 modified American College of Rheumatology classification criteria, had a baseline SLEDAI of ≥6, and had been scored using BILAG, SLEDAI, and physician’s global assessment (PGA) at two study visits. Some PGA values were retrospectively assessed, if PGA had not been measured at the time of study visit, and clinical changes were compared with baseline, assessed as physician-rated improvement (PRI). Changes to medication were also measured from baselines to both study follow-up visits. Patients were assessed between 2009 and 2012.
Results
In total, 91 patients with SLE were included in this analysis. The mean age at baseline was 41 years. As measured by PRI, 68 patients improved, 17 stayed the same, and 6 patients deteriorated. Improvement and deterioration were also measured using PGA, SLEDAI, and BILAG, and compared to PRI. Disease activity as measured by PGA, SLEDAI, and BILAG in patients who were found to have improved by PRI is shown in Figure 2.
Figure 2. Change in disease activity from baseline to follow up in PRI responders*
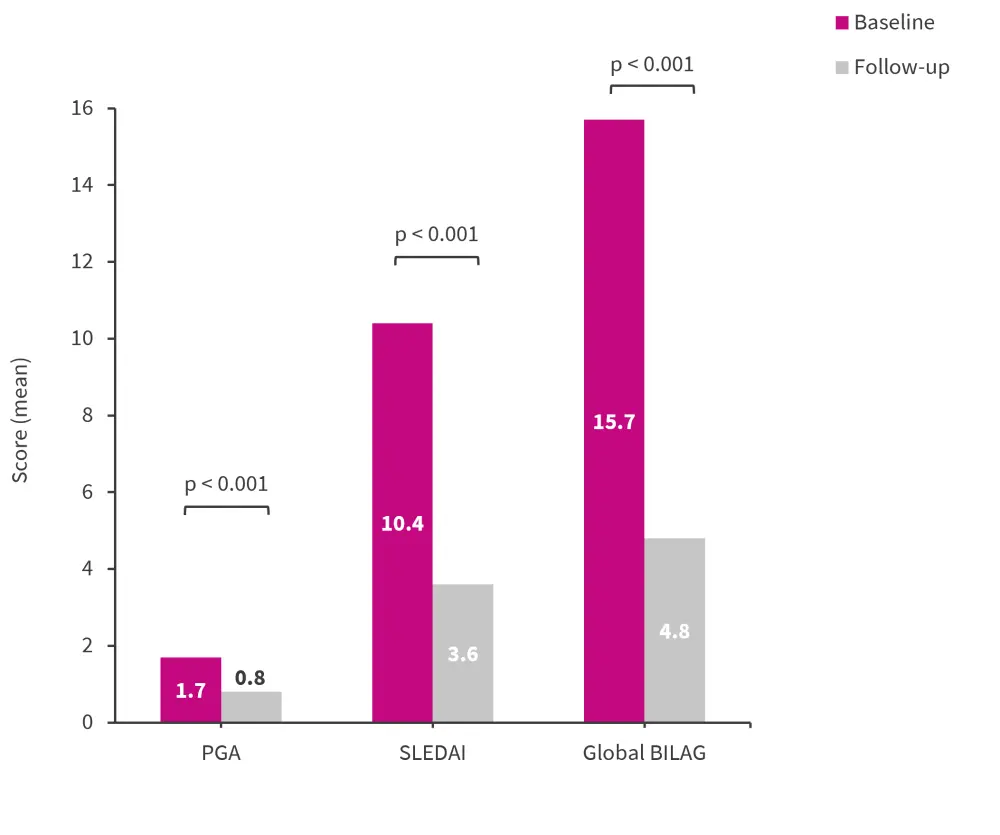
BILAG, British Isles Lupus Assessment Group; PGA, physician’s global assessment; PRI, physician-rated improvement; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index.
*Data from Thanou, et al.1
SRI and BICLA accuracy
Of the 68 patients who were found to have improvements by PRI, 58 met the SRI endpoint (agreement sensitivity with PRI, 85%) and 52 met the BICLA endpoint (agreement sensitivity with PRI, 76%). Of the 23 patients who stayed the same or deteriorated according to PRI, 17 and 23 did not meet the SRI and BICLA endpoints, respectively, with a specificity of SRI for PRI of 74% and BICLA for PRI of 78%.
Ten patients did not meet SRI despite improvements in PRI, of these patients seven met the BICLA endpoint. Further, of the 16 patients who improved in PRI but not BICLA, 13 improved by SRI. This highlights the variable success of endpoints in identifying improvement. It was also found that a BILCA response was less likely when a greater number of organs were active at baseline.
Study conclusions
This study demonstrated that BICLA may be less sensitive than SRI in detecting improvement, when compared to PRI. It is important to note that this comparison does not consider all patient populations or background treatments, which may influence the accuracy of these measures in other clinical trials.
New approaches2
Newly developed and novel outcome measures, which can measure changes to response, are needed. Newer approaches in various stages of development include:
- The SLEDAI-2K Responder Index 50 is based on SLEDAI-2K and defines a 50% improvement threshold for each manifestation. This measure has been demonstrated as more effective at detecting patients who may experience incomplete responses than the original SLEDAI; however, measuring improvement in subjective manifestations can still create difficulties.
- The Lupus Multivariable Outcome Score was created using data from the phase III belimumab trials, for learning and validation, and uses elements of SLEDAI and BILAG to calculate a score. It is unknown how well this measure will perform in different trial populations.
- The Lupus Foundation of America’s Rapid Evaluation of Activity in Lupus uses visual analog scales in which the physician can score organ-specific and global disease activity continuously.
- The SLE Disease Activity Score is a continuous measure that uses multivariate linear regression, with PGA as the dependent variable, to produce a weighted score of 17 variables. This has shown increased sensitivity when compared to SLEDAI.
- The Cutaneous Lupus Activity and Severity Index is an organ specific measure. Use of single measures such as this have shown success in lupus nephritis trials.
- It has been suggested that patient reported outcomes could be used to measure the success of new treatments but would have limited scope as a standalone primary outcome measure in clinical trials.
Conclusion
There is a need for improved methods of measuring efficacy in SLE clinical trials, with many promising therapies failing to proceed past phase III trials.1 Ideally, future outcomes can be developed to take both physician and patient reported measures into account which are simple and reliable.2 Reliance on PGA and BILAG, which may lack consistency due to the subjective nature of the measurements, is not sufficient to properly define SLE disease activity.2 In addition, SRI and BICLA have been shown to miss improvements or deteriorations in disease, when compared with PRI.1 Finally, outcome measures used for clinical trials should be considered on an individual basis, based on the study design and patient population enrolled.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



