All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Editorial theme | Treat-to-target in SLE
Do you know... Lupus Low Disease Activity State (LLDAS) is seen as a more attainable treatment target for patients with SLE. The definition of LLDAS involves multiple components. Which statement is not a criterion for LLDAS?
High rates of remission with novel targeted agents have been achieved with many rheumatic diseases; however, treatment of systemic lupus erythematosus (SLE) remains difficult. While there are clinical trials underway to investigate novel therapies, the final efficacy results remain awaited. In the interim, treatment outcomes could be optimized for patients with SLE by adopting specific treatment strategies, such as the treat-to-target (T2T) strategy, that prevent damage accrual and reduce flares and mortality.1
In this first article in our editorial theme on treatment goals, we examine the T2T strategy and consider the advantages and disadvantages of its use when managing patients with SLE. Further information on the clinical considerations for SLE can be found in our related article here.
EULAR/ACR recommendations on treatment goals2
The 2019 European Alliance of Associations for Rheumatology (EULAR)/American College of Rheumatology (ACR) recommendations state that the goals of treatment should be to aim for remission or low disease activity and prevention of flares in all organs, maintained with the lowest possible dose of glucocorticoids. The guidelines state that achieving complete remission is infrequent and suggests the use of Lupus Low Disease Activity State (LLDAS) as a treatment goal to allow a comparable decrease in damage accrual and prevention of flares.
For further information on the EULAR/ACR recommendations for management of SLE, please see our related article here.
What is T2T?1
T2T is a strategy that has been used successfully in other diseases, including diabetes and rheumatoid arthritis. The T2T strategy involves four steps, as outlined in Figure 1.
Figure 1. T2T strategy for the treatment of SLE*
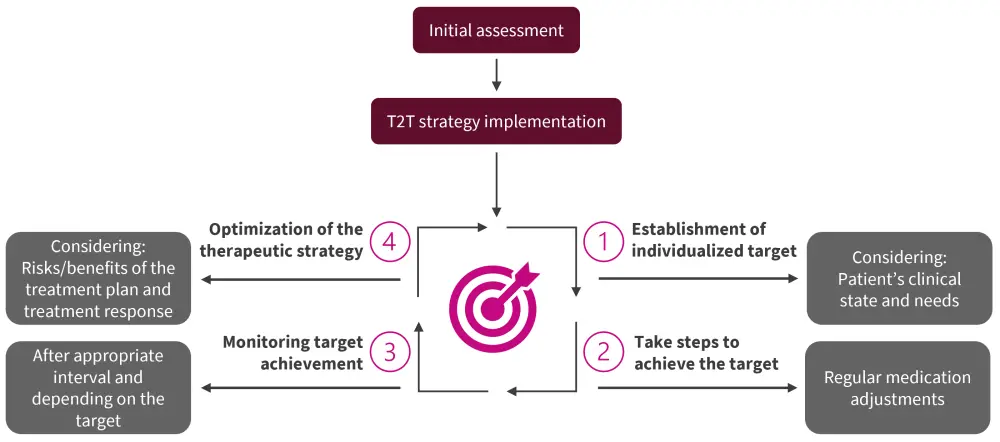
SLE, systemic lupus erythematosus; T2T, treat-to-target.
*Adapted from Parra Sánchez, et al.1
For SLE, monitoring intervals are suggested of 3−6 months, while assessing disease activity, response to treatment, and damage (both from disease and as a result of therapy).
T2T has been suggested as an effective strategy for the treatment of SLE, although this has yet to be formally assessed in a clinical trial against standard care. To make T2T a viable option, defined treatment goals are needed along with therapeutic options to achieve these goals.
T2T endpoints1
The main goal when treating SLE is to achieve remission, ideally without immunosuppressive agents and no serological activity. However, this can be very challenging to achieve in some patients with the current therapeutic options available. LLDAS is considered as an attainable intermediate goal in the SLE T2T strategy and the benefits of attaining and maintaining LLDAS in patients have been shown in a several studies, such as the Hopkins Lupus Cohort and the Asia-Pacific collaborative study.
The Definition of Remission in SLE (DORIS) is more stringent than LLDAS, with a SLE Disease Activity Index (SLEDAI) index score of 0 being necessary, whereas LLDAS allows up to 4 points. The maximum dose of glucocorticoids is also lower in DORIS at <5 mg daily prednisolone compared with ≤7.5 mg daily prednisolone in LLDAS (Table 1).
Table 1. Definitions of the T2T endpoints LLDAS and DORIS*
|
cSLEDAI, Clinical SLE Disease Activity Index; DORIS, Definition of Remission in SLE; LLDAS, Lupus Low Disease Activity State; PGA, Physician’s Global Activity; SLE, systemic lupus erythematosus; SLEDAI-2K, SLE Activity Index 2000; T2T, treat-to-target. |
|
|
Treatment goal |
Definition |
|---|---|
|
LLDAS |
SELENA SLEDAI-PGA (scale, 0–3) score ≤1 |
|
SLEDAI-2K score ≤4, with no activity in major organ systems (including renal, central nervous system, cardiopulmonary, vasculitis, and fever) and no hemolytic anemia or gastrointestinal activity |
|
|
Current prednisolone (or equivalent) dose ≤7.5 mg daily |
|
|
Standard maintenance doses of biologics and immunosuppressive drugs |
|
|
No new features of lupus disease activity (according to SLEDAI-2K) compared with the previous assessment |
|
|
DORIS |
PGA (scale 0–3) score <0.5 |
|
cSLEDAI = 0 |
|
|
The patient may be on antimalarial, prednisolone <5 mg daily (or equivalent), and/or stable immunosuppressive drugs, including biologics |
|
|
Status independent of serology results |
|
The difference between the various SLEDAI tools
DORIS and LLDAS are evaluated using several different tools, including the SLE Disease Activity Index (SLEDAI), which has different iterations from its use in different studies.3
- The classical SLEDAI evaluates lupus disease activity in the 10 days prior to the assessment. It uses 24 weighted clinical and laboratory variables from nine organ systems. Scores between 1 and 8 are given and the maximum possible score for all variables is 105.3
- SLEDAI‑2K is a modified version of the original index that allows for documentation of rash, alopecia, mucosal ulcers, and proteinuria in the descriptors. SLEDAI‑2K has been compared with the classical SLEDAI and was shown to indicate changes over time. A 30-day extended version has been assessed and been shown to be equivalent to the standard 10-day SLEDAI‑2K.3
- Clinical SLEDAI, or clinical SLEDAI-2K (cSLEDAI or cSLEDAI-2K), is a variant of the index that omits the anti-dsDNA and complement levels.4
- Safety of Estrogens in Lupus National Assessment (SELENA)-SLEDAI was developed for the SELENA study, which investigated the use of oral contraceptives in patients with SLE.5 This version also allowed modified scoring to indicate whether an item (such as rash, mucosal ulcers, and alopecia) was persistent and active, and added a glossary.3
Benefit of using T2T1
The effect on outcomes, such as health-related quality of life (HRQoL), survival, and prevention of long-term organ damage, using LLDAS and DORIS has been investigated in several studies. In the Asia-Pacific Collaborative study, patients who spent >50% of their time in LLDAS demonstrated a significant reduction in the cumulative organ damage and had a relative risk of 0.47 (p = 0.005) of having ≥1 increase in the Systemic Lupus International Collaborating Clinics (SLICC)/ACR Damage Index.
DORIS has also been shown to be associated with decreased organ damage, flares, and improved patient QoL; therefore, either endpoint is thought to be suitable as a treatment goal for patients with SLE.
Impact on patient-reported outcomes6
Achieving remission in SLE is associated with an improvement in HRQoL, which is a key endpoint from a patient’s perspective. Two cohort studies found that long-term remission ≥5 years was associated with improvements in HRQoL when using the SF-36 and LupusPRO tools. Using LLDAS as an endpoint has also shown similar results.
Treatments facilitating T2T1
Recent advances in the understanding of SLE pathogenesis have enabled the development of targeted therapies for patients who respond inadequately to conventional treatments, making T2T feasible in routine patient management. An overview of SLE management has previously been published on the Lupus Hub, here.
Challenges in adopting T2T in clinical practice1
Patients may be resistant to the T2T strategy if the rationale behind it is not properly explained as it may involve an increased number of visits to the clinic for monitoring purposes and more frequent changes of medication. This could potentially lead to reduced trust in the primary physician and increased non-compliance with prescribed medication. In addition, treatments that are achieving moderate success in treating SLE may be stopped in favor of a less successful agent under the T2T model as the primary endpoint was not met. This approach may also lead to the use of more expensive medication more quickly than standard of care if an adequate response is not attained. As treatment options for SLE remain limited this may lead to rapid exhaustion of treatment options.
T2T trials1
As the T2T strategy has not yet been compared against standard of care in a prospective study, an ongoing study, the Lupus-BEST trial (NCT05714930), aims to assess the impact on organ damage accrual and improve HRQoL. The trial will consist of three arms; standard of care, T2T with a goal of DORIS, and T2T with a goal of LLDAS. The design of this trial should allow assessment of which endpoint is feasible, along with calculation of the benefit-risk ratio of each treatment arm and the impact on adverse events and organ damage.
Conclusion
SLE is a heterogenous disease that makes treatment challenging. However, the adoption of clinical strategies such as T2T has the potential to improve not only the number of flares and decrease the amount of damage accrual but also improve a patient’s HRQoL. With several novel therapies on the horizon, T2T strategies for SLE management are more viable as there are sufficient therapeutic options for patients. LLDAS is the more clinically achievable treatment goal currently, but with the advent of novel agents for the treatment of SLE, DORIS may become more achievable for a larger group of patients. Despite the challenges of implementing the T2T strategy in the clinic, it has the potential to greatly improve treatment outcomes for patients and upcoming trials should provide further information on the benefits and caveats of using this strategy.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


