All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Povetacicept: An enhanced dual BAFF/APRIL inhibitor for treatment of SLE
B-cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL) signal through B-cell maturation antigen (BCMA), TACI (transmembrane activator and CAML interactor), and/or BAFF-receptor plays important role in the pathogenesis of systemic lupus erythematosus (SLE). Povetacicept, a potent enhanced dual BAFF/APRIL inhibitor, has demonstrated efficacy, enhanced serum exposure, and pharmacodynamics in previous preclinical studies.
At the 14th European Lupus Meeting, Simsek.1 presented the analysis of published transcriptional datasets to ascertain the gene expression of BAFF and APRIL in patients with SLE. A comparison of povetacicept to wild-type (WT) TACI-Ig in tissue distribution and SLE disease activity in murine models was also discussed.1 Here, we summarize the key findings.
Methods
-
Publicly available RNA-Seq datasets from healthy donors and patients with SLE were used to assess the gene expression of APRIL and BAFF.
-
In vivo biodistribution was assessed by intravenously injecting povetacicept (10 mg/kg) or matching dose of WT TACI-Ig (telitacicept) to healthy C57BL/6 mice. Lupus-related tissues were collected after 18 hours, and quantitative immunohistochemistry was performed for human Fc staining.
-
The study objectives were to ascertain the expression of BAFF and APRIL in patients with SLE using transcriptional datasets; and to compare povetacicept to WT TACI-Ig in tissue distribution and lupus efficacy using mouse models.
-
Povetacicept was also tested in an interferon-α-accelerated NZB/W mouse model of SLE, compared with matched Fc control, WT TACI-Ig, a depleting anti-mouse CD20 mAb (anti-mCD20), and cyclophosphamide.
Key findings
-
In the transcriptional datasets, genes related to BAFF (TNFSF13B), APRIL (TNFSF13), BAFF-receptor (TNFRSF13C), BCMA (TNFRSF17), and TACI (TNFRSF13B) were increased in myeloid lineage cells and B cells in patients with SLE compared with healthy adults.
-
Povetacicept, compared with WC TACI-Ig, is smaller in size (molecular weight: 62.6 kDA vs 75.3–75.4, respectively), has a lower isoelectric point (6.5–7.0 vs 7.1–8.4, respectively), and higher target affinity. Consequently, povetacicept exhibited greater distribution to lupus-related organs in normal mice than WT TACI-Ig (Figure 1).
Figure 1. Distribution of povetacicept and WT TACI-Ig to lupus-related organs*
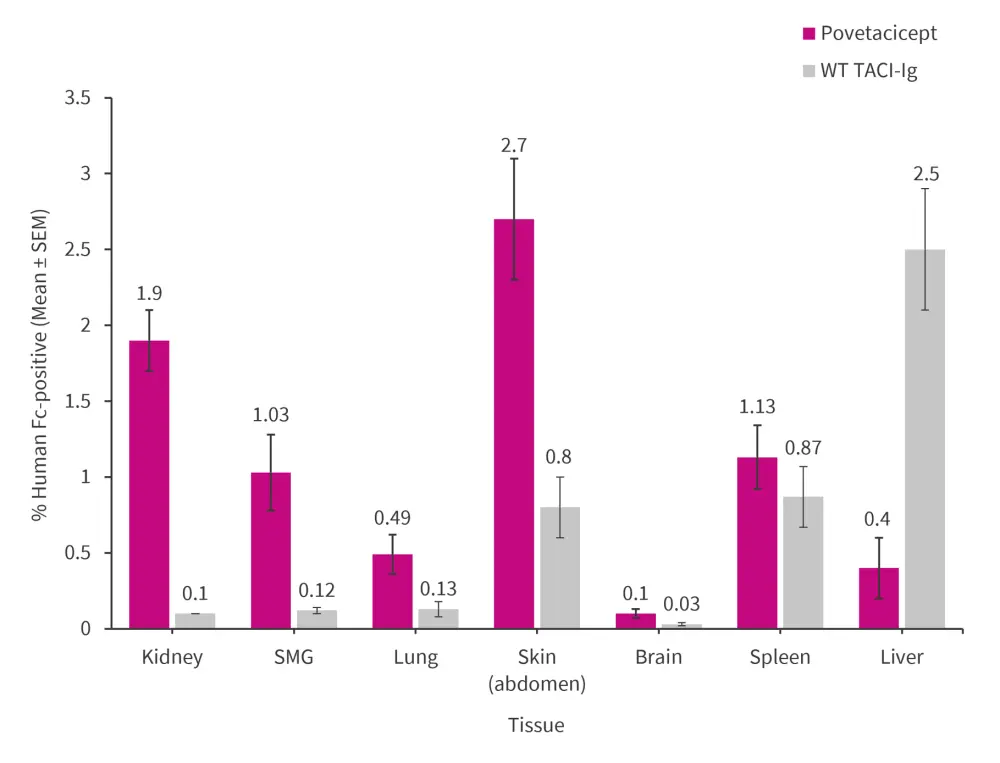
TACI, transmembrane activator and CAML interactor; SEM, standard error of the mean; WT, wild-type.
*Data from Simsek.1
Flow cytometric analysis of spleens collected at Day 50 revealed that povetacicept:
-
-
reduced the frequency of total B cells (CD19+B220+CD11b-), plasma cells (live TACI+CD138+ cells), and anti-double stranded DNA as compared with Fc control, anti-mCD20, or WT TACI-Ig groups; and
-
increased hemoglobin levels as compared with Fc control, WT TACI-Ig, or cyclophosphamide groups.
-
-
In the histopathology analysis, povetacicept showed a significant reduction in:
-
-
proteinuria scores on Day 48 and total lesion scores (glomerular + tubular/interstitial lesions) in kidney, as compared with Fc control and WT TAC-Ig;
-
glomerular IgG deposition, compared with Fc control, anti-mCD20, and WT TACI-Ig; and
-
numbers of inflammatory foci in submandibular gland, compared with Fc control and anti-mCD20.
-
|
Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

