All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Role of Smith-specific regulatory cells in the management of lupus nephritis
Antigen-specific regulatory T cells (Tregs) are implicated in the pathogenesis of systemic lupus erythematosus (SLE). Lupus nephritis (LN), a severe manifestation of SLE, is strongly associated with autoreactivity to the Smith (Sm) autoantigen and the human leucocyte antigen (HLA)-DR15 haplotype.
To explore the therapeutic opportunity of these associations, Eggenhuizen et al. published an article in Nature Communications, investigating the potential of Sm-specific Tregs (Sm-Tregs) to suppress LN.1 Here, we summarize the key insights.
Methods1
- Sm HLA-DR15 restricted CD4+ T-cell epitopes were discovered using biophysical affinity assays.
- High throughput single-cell sequencing was used to identify highly reactive Sm-specific T-cell receptors (TCR).
- Using lentiviral vectors, Sm TCR was transduced into Tregs obtained from patients with SLE who were anti-Sm+ and HLA-DR15+, to test the suppressive function of Sm-Tregs in vitro and in vivo.
Key findings1
- SmB/B’58-72 appeared as the dominant T-cell epitope in LN, exhibiting the highest binding and greatest stability to HLA-DR15.
- The protein crystal structure of SmB/B’58-72 complexed with HLA-DR15 confirmed that SmB/B’58-72 binds HLA-DR15.
- TCR1 showed the strongest affinity for SmB/B’58-72, and induced T-cell activation and memory.
- Sm-Tregs maintained their regulatory phenotype. Following 10 days of in vitro expansion:
- 98% of CD4+ Sm-Tregs were CD25hiCD127lo and 87% of the Sm-specific Tregs coexpressed the Treg-specific transcription factors Foxp3 and Helios
- Further, Sm-Tregs showed consistent Treg-cell-specific demethylated region long-term, a hallmark of stable Treg phenotype
- Additionally, Sm-Tregs showed low expression of proinflammatory cytokines interleukin (IL)-17A and interferon (IFN)-γ
- In the presence of SmB/B’58-72 autoantigen, Sm-Tregs showed increased inhibition of Sm-CD4+ T-conventional cells, compared with mock Tregs and no Tregs.
- Co-culture supernatant with Sm-Tregs showed a significantly higher concentration of IL-10 but decreased IFN-γ and IL-17A compared with polyclonal Tregs and no Tregs (Figure 1).
Figure 1. Sm-Tregs suppress autoimmunity in samples from patients with SLE in vitro*
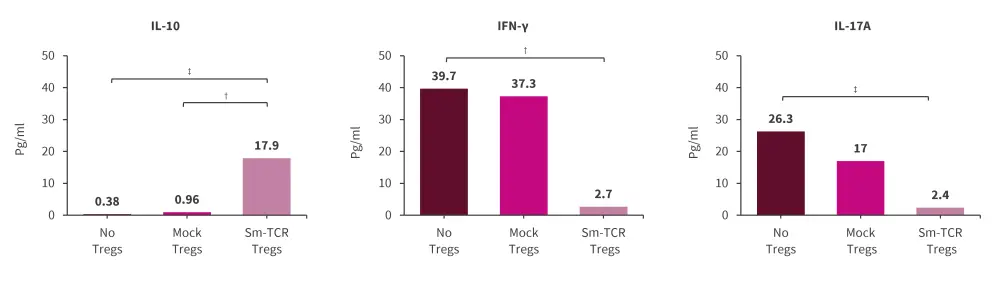
IFN, interferon; IL, interleukin; SLE, systemic lupus erythematosus; Sm, Smith; TCR, T cell receptor; Treg, regulatory T cells.
Data are presented as mean.
*Adapted from Eggenhuizen, et al.1
†p < 0.0332.
‡p < 0.0021.
- In a humanized mice model, patient-derived SM-Tregs significantly reduced proteinuria, percentage of necrotized glomeruli, glomerular hypercellularity, mesangial cell proliferation, and tubulointerstitial injury, compared with mice administered with polyclonal Tregs and no Tregs (Figure 2).
Figure 2. Sm-Tregs suppress disease in humanized mouse models of LN*
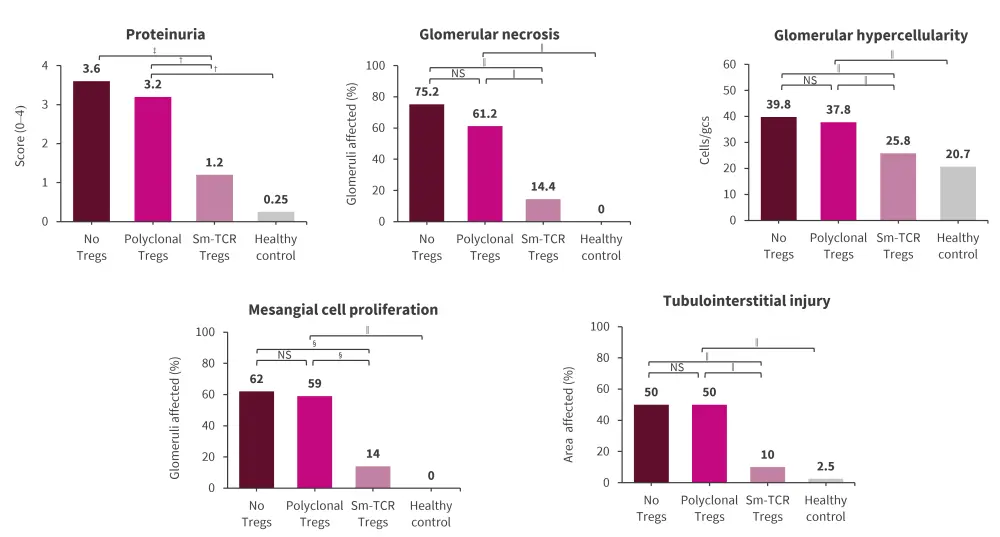
LN, lupus nephritis; NS, non-significant; Sm, Smith; TCR, T-cell receptor; Treg, regulatory T cells.
*Adapted from Eggenhuizen, et al.1
†p < 0.0332.
‡p < 0.0021.
§p < 0.0002.
‖p < 0.0001.
Data are presented as mean.
|
Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

