All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Safety and efficacy of itolizumab in lupus nephritis: results from phase Ib EQUALISE study
Itolizumab is a first-in-class anti-CD6 monoclonal antibody that selectively targets the CD6-activated leukocyte cell adhesion molecule signaling pathway to suppress pathogenic T effector cells while maintaining T regulatory cells. Itolizumab has recently been evaluated in an open-label, proof-of-concept, phase Ib EQUALISE study, showing a favorable safety and tolerability profile in patients with lupus nephritis.
Topline results1
- Out of 17 patients enrolled, 16 patients completing Week 36 were analyzed.
- The baseline mean urine protein creatinine ratio (UPCR) was 4.9 g/g.
- The median spot UPCR showed a reduction of ~73% from the baseline.
- By Week 36, more than 80% of subjects achieved >50% reduction in UPCR (Figure 1)
Figure 1. Clinical response*
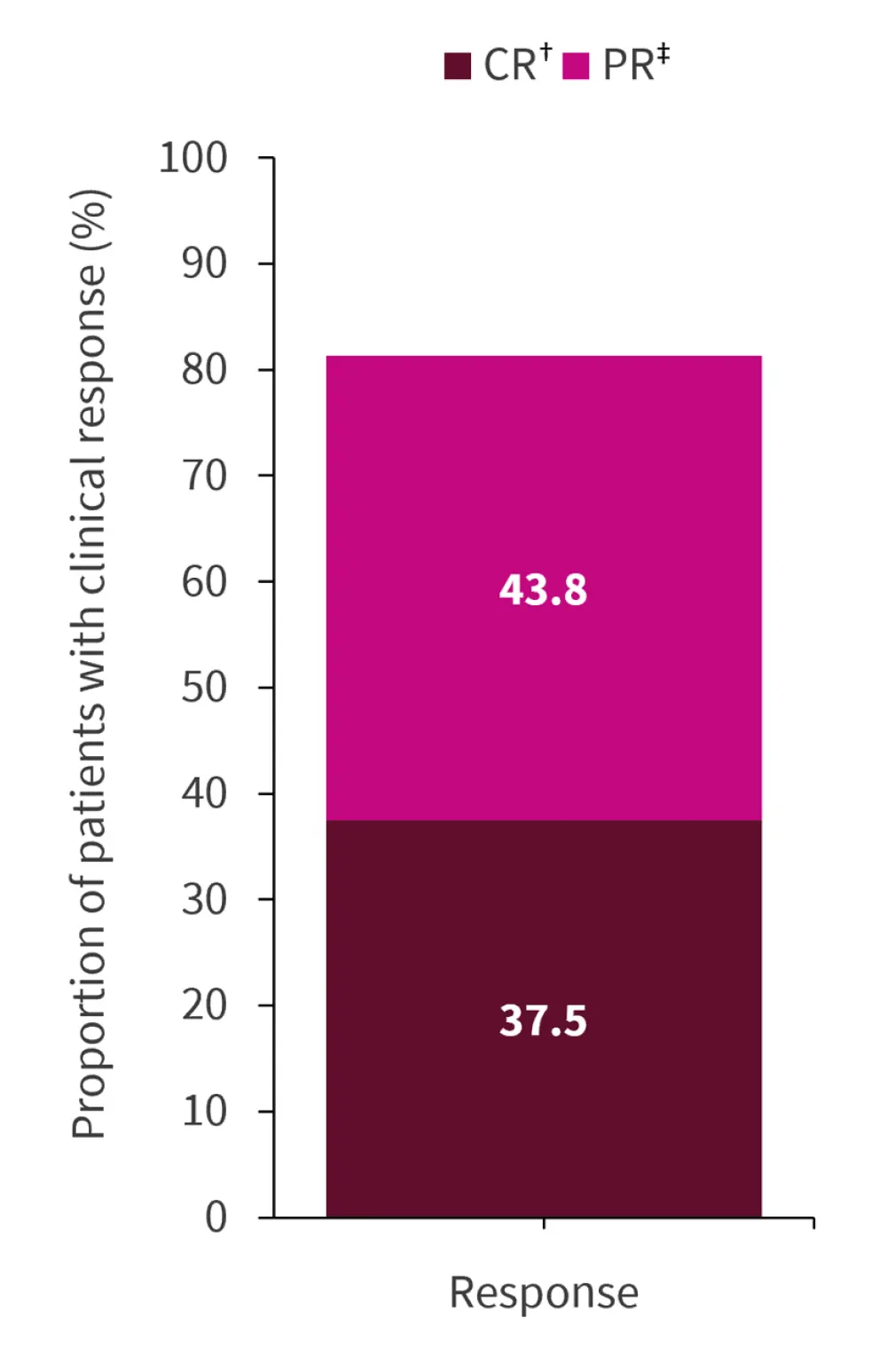
CR, complete response; PR, partial response.
*Data from Businesswire.1
†UPCR ≤0.7 g/g.
‡UPCR ≥50% reduction.
- The overall response rate (ORR) achieved in patients receiving itolizumab by Week 12 and 28 were greater than the ORR in patients receiving standard-of-care alone. Additionally, the results were comparable with those from voclosporin in the phase III AURORA1 study (ORR 70% at Month 6 and 12 in active treatment).
- Patients were able to taper their systemic corticosteroids by >80% by Week 24.
- Itolizumab consistently reduced cell surface CD6 levels on T cells and was associated with reductions in absolute lymphocyte counts over a 6-month treatment period.
- Reduction in absolute lymphocyte counts was not associated with increased rates of infection or other adverse events.
- The majority of treatment-emergent adverse events were mild (Grade 1) or moderate (Grade 2) in severity. The two most common treatment-emergent adverse events were lymphopenia and peripheral edema. Two subjects had ≥1 serious adverse event, unrelated to study treatment.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

