All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Safety and efficacy of voclosporin-based triple immunosuppressive therapy in lupus nephritis
Voclosporin, a second-generation calcineurin inhibitor, is approved for the treatment of adults with active lupus nephritis, alongside a standard immunosuppressive regimen.1 The phase II AURA-LV and phase III AURORA 1 trials used a combination of voclosporin with mycophenolate mofetil (MMF) and oral glucocorticoids (GCs).1 An integrated analysis of AURA-LV/AURORA 1 demonstrated that voclosporin-based triple immunosuppressive therapy led to significantly greater and earlier reductions in proteinuria, with an acceptable safety profile compared with placebo.1
At the 14th European Lupus Meeting, Dall’Era et al. presented a post hoc analysis comparing safety and efficacy of voclosporin-based triple immunosuppressive therapy from the AURA-LV and AURORA 1 trials to intravenous cyclophosphamide (IVC) and MMF based double immunosuppressive therapy (IVC/MMF + high-dose GCs) from phase III ALMS trial.1 We summarize the key findings below.
Methods1
-
Propensity matching identified groups of matched participants (ALMS vs AURA-LV/AURORA 1) with similar demographic and disease characteristics.
-
Safety and efficacy were assessed at 3 and 6 months.
Key findings1
-
A total of 179 matched pairs were identified
-
-
From the ALMS trial, 91 patients received IVC + GCs and 88 received MMF + GCs
-
-
Mean cumulative oral GC exposure was two-fold lower in the voclosporin vs IVC and MMF groups over both 3 and 6 months (Figure 1).
-
At 6 months, 79.9%, 8.8%, and 5.7% of patients in voclosporin, IVC, and MMF groups, respectively achieved a GC dose of ≤7.5 mg/day.
Figure 1. Cumulative glucocorticoid exposure with immunosuppressive therapies*
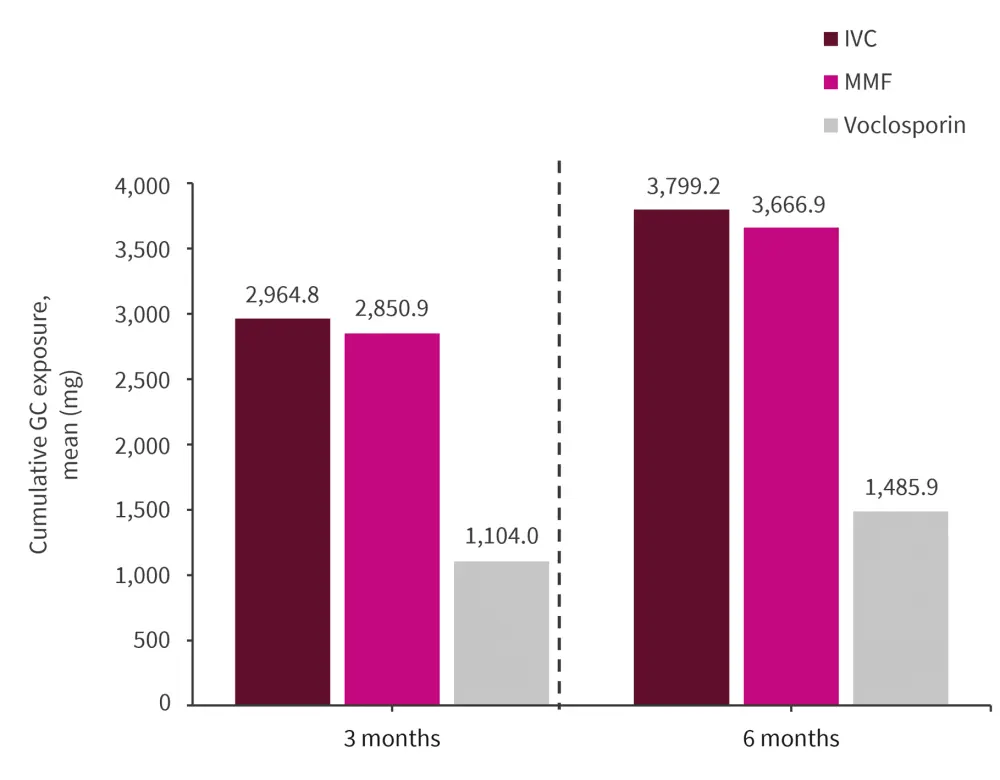
GC, glucocorticoid; IVC, intravenous cyclophosphamide; MMF, mycophenolate mofetil.
*Data from Dall’Era, et al.1
-
Overall incidence of adverse events was lower in voclosporin compared with IVC-and MMF groups (Figure 2).
-
However, at 6 months a higher proportion of patients in the voclosporin group compared with IVC and MMF groups reported decreased glomerular filtration rate (24.6% vs 0% and 0%, respectively) and hypertension (17.3% vs 12.1% and 14.8%, respectively).
Figure 1. Adverse events at 3 and 6 months*
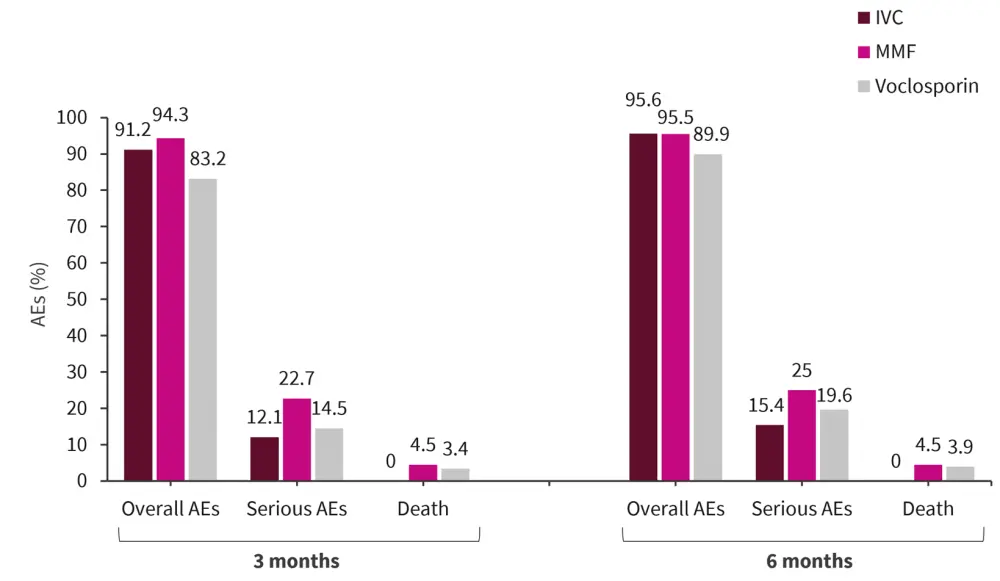
AE, adverse event; IVC, intravenous cyclophosphamide; MMF, mycophenolate mofetil.
*Data from Dall’Era, et al.1
- At 3 months, a higher proportion of patients in the voclosporin group achieved a 25% reduction in urine protein creatinine ratio (91.6%) than IVC (61.5%; p < 0.0005) and MMF (77.3%; p < 0.005) groups.
- Likewise, a 50% reduction in urine protein creatinine ratio at 6 months was significantly higher in with voclosporin group (70.9%) than IVC (57.1%; p < 0.05) and MMF (56.8%; p < 0.05) groups.
|
Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

