All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Safety of enpatoran in patients with active SLE/CLE: results from a phase Ib study
At the 14th European Lupus Meeting, Roy.1 presented safety findings from an ongoing phase Ib study of enpatoran by Wenzel et al.2 in patients with active SLE or cutaneous lupus erythematosus (CLE).1,2 Here, we summarize the key points.
Study design
-
This is a randomized, placebo-controlled, double-blind, dose-ascending trial (NCT04647708), exploring the maximal anticipated therapeutic range of oral enpatoran vs placebo in patients with active SLE/CLE (Figure 1).1,2
Figure 1. Study design*
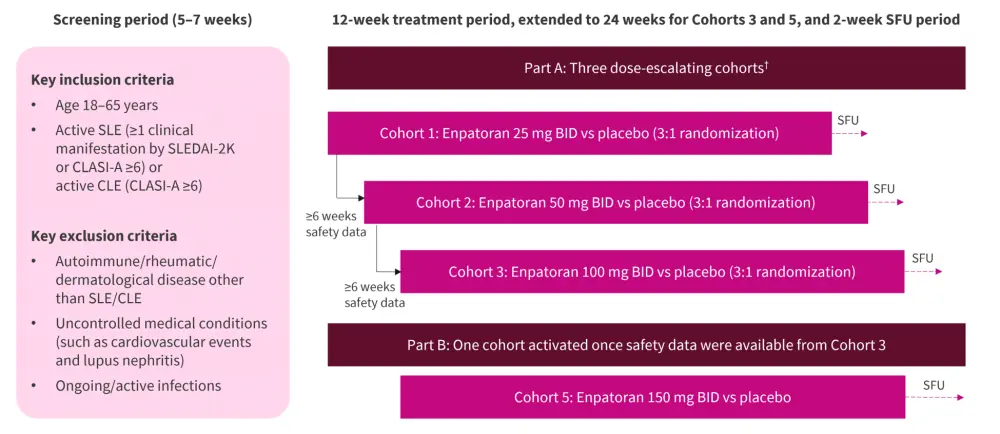
CLASI, Cutaneous Lupus Erythematosus Disease Area and Severity Index; CLE, cutaneous lupus erythematosus; BID, twice-daily; SFU, safety follow-up; SLE, systemic lupus erythematosus; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000.
*Adapted from Roy.1
†Cohort 4 was optional and was not activated.
-
This analysis was based on blinded data with a cut-off date of Aug 10, 2023.2
Key findings1,2
-
Out of 25 randomized patients, 20 completed treatment.
-
-
Two patients were undergoing treatment, while three had discontinued treatment due to reasons unrelated to safety.
-
-
Overall, 12 patients reported ≥1 treatment-emergent adverse event (TEAE; Figure 1).
-
-
93% of TEAEs were mild (n = 19) or moderate (n = 8) in severity.
-
One Grade 4 TEAE (laboratory abnormality of decreased lymphocyte count) was reported.
-
-
There were no instances of Grade 3 TEAEs, serious TEAEs, dose-limiting toxicities, adverse events of special interest, life-threatening TEAEs, or deaths (Figure 2).
-
No safety concerns were observed in the electroencephalogram and electroencephalogram findings.
-
-
No clinically significant abnormalities were noted in electrocardiogram readings.
-
All electroencephalogram results were within the normal limits.
-
Figure 2. Safety findings*
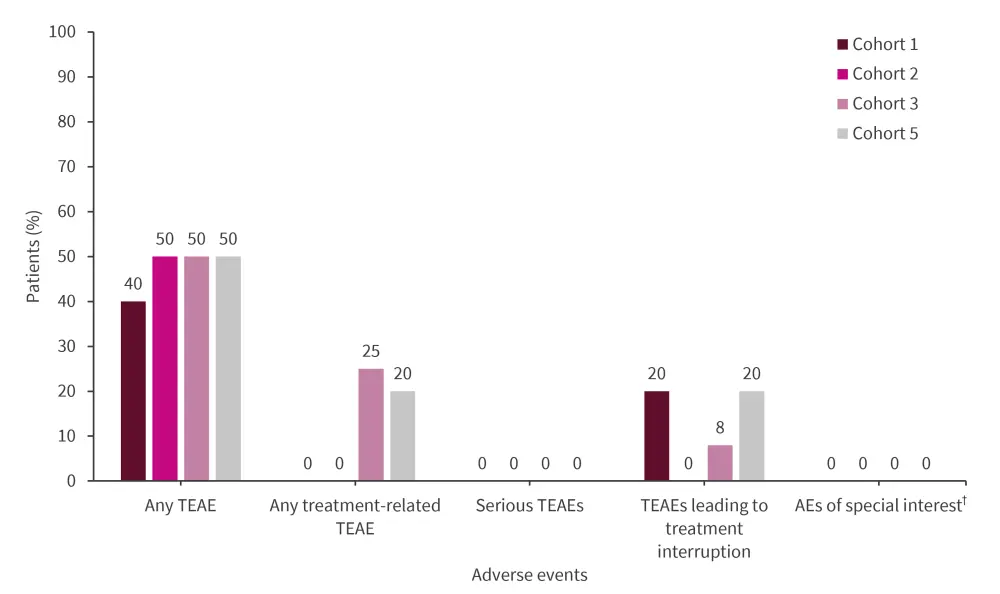
AE, adverse event; TEAE, treatment-emergent AE.
*Data from Roy.1
†Includes severe infections, seizures, serotonin syndrome, and clinically significant cardiac arrhythmias.
|
Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

