All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
SLE-DAS as a disease activity measure: Post hoc analyses of phase II and III trials of anifrolumab
Accurate and practical disease activity measures in the management of systemic lupus erythematosus (SLE) are limited.1 The SLE Disease Activity Score (SLE-DAS) is a recently validated 17-item tool that demonstrates high accuracy and sensitivity in assessing changes to disease activity.2
At the 14th European Lupus Meeting, Jesus presented post hoc analyses of phase II and III trials of anifrolumab to:
- derive and validate the cut-off value for SLE-DAS for defining severe disease activity (SDA);1 and
- evaluate if SLE-DAS enables easy identification of patients with SDA and moderate-to-severe disease activity (MSDA), and with worse health-related quality of life (HR-QoL). 1,2
Here, we summarize the key findings.
Methods1,2
- These were post hoc analyses of aggregated intention-to-treat data from placebo arms in MUSE (NCT01438489), TULIP-1 (NCT02446912), and TULIP-2 (NCT02446899) trials on anifrolumab vs placebo in moderate-to-severe SLE.
- British Isles Lupus Assessment Group (BILAG-2004) Index, SLE Disease Activity Index 2000 (SLEDAI-2K), and the patient-reported outcomes ([PROs]: lupus quality of life, EuroQoL-5D, Functional Assessment of Chronic Illness Therapy – Fatigue, and Patient Global Assessment) were assessed. The SLE-DAS was retrospectively scored at each visit.
- The Mann-Whitney test was used to compare PROs between patients in SDA vs non-SDA and MSDA vs non-MSDA at Week 12 and Cohen’s d was used to compare the magnitude of these differences.
Key findings
SDA1,2
- A total of 438 patients with SLE were included.
- At Week 12, 46.6% in the derivation cohort and 42.4% in the validation cohort were in SDA by BILAG-2004.
- SLE-DAS >9.90 (area under curve = 0.847) was the best cut-off to identify patients in SDA in the derivation cohort, which showed 77.8% sensitivity and 79.6% specificity in the validation cohort.
- Patients in SDA by SLE-DAS reported significantly worse impact in all HR-QoL PROs (Figure 1).
MSDA2
- Of 438 patients identified as MSDA by BILAG-2004, 96.1% were classified as MSDA by SLE-DAS, and 92.9% by SLEDAI-2K.
- At Week 12, SLE-DAS and SLEDAI-2K presented significantly worse impact in all HR-QoL PROs (Figure 1).
Figure 1. HRQoL comparison between patients in A SDA vs non-SDA1 and B MSDA vs non-MSDA2, according to SLE-DAS, BILAG-2004, and SLEDAI-2K*
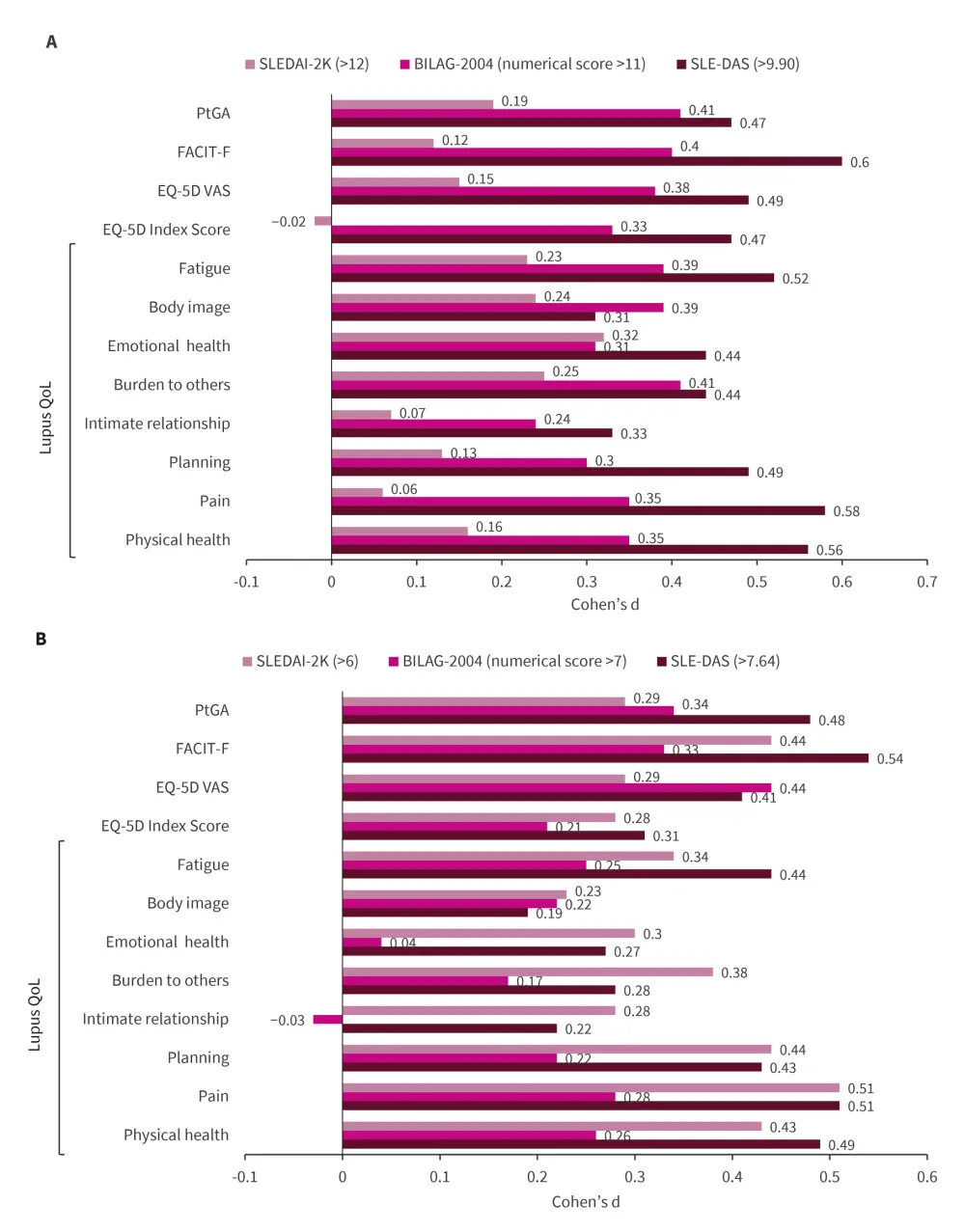
BILAG, British Isles Lupus Assessment Group; EQ-5D, EuroQoL-5D; FACIT-F, Functional Assessment of Chronic Illness Therapy – Fatigue; MSDA, Moderate-to-Severe Disease Activity; PtGA, Patient Global Assessment; QoL, quality of life; SDA, Severe Disease Activity; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000; VAS, visual analog scale.
*Data from Jesus, et al.1,2
|
Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

