All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Tapering steroids in modified serologically active clinically quiescent patients with SLE
Serological activity in systemic lupus erythematosus (SLE) is characterized by the presence of anti-double-stranded DNA antibodies and/or hypocomplementemia. Some patients with SLE exhibit persistent serological activity, despite clinical quiescence. Appropriate use of glucocorticoid (GC) therapy and withdrawal in these serologically active, clinically quiescent (SACQ) patients needs to be investigated.
Katsumata et al. recently published an article in Annals of the Rheumatic Diseases assessing the risk of flare and damage accrual after tapering GCs in patients with modified SACQ (mSACQ; SACQ not-considering duration) with SLE.1 Here, we summarize their key findings.
Methods1
- Data were collected prospectively from a large multinational longitudinal cohort between 2013 and 2020.
- The primary outcome was disease flare and the secondary outcome was irreversible organ damage accrual (any increase in Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index [SDI] score).
- Cox proportional hazard models were used to assess the risk of subsequent flare or damage accrual per 1 mg decrease of prednisolone-equivalent GC dosages.
- Each initial dosages of prednisolone-equivalent GC were analyzed:
- 0≤ and ≤5 (relevant to remission);
- 0≤ and ≤7.5 (relevant to Lupus Low Disease Activity State); and
- >5 and ≤7.5 (relevant to Lupus Low Disease Activity State but not in remission).
Key findings1
- A total of 1,850 patients were studied, with 2 years of subsequent follow-up data since their initial mSACQ visit.
Flare
- Of 1,850 patients, 742 patients experienced overall flare and 271 experienced severe flare.
- Tapering GCs showed no association with an increased risk of subsequent overall or severe flare in any dosage groups (Figure 1).
- In contrast, among patients with initial prednisolone-equivalent GC dosages of 0–7.5 or 0–5 mg/day, antimalarial use was significantly associated with decreased risk of:
- overall flare: hazard ratio (HR), 0.78; 95% confidence interval (CI), 0.66–0.91; p = 0.002; and
- severe flare: HR, 0.59; 95% CI, 0.46–0.76; p < 0.001.
Figure 1. Risk of A overall flare, B severe flare, and C damage accrual when tapering GCs in mSACQ patients with SLE*
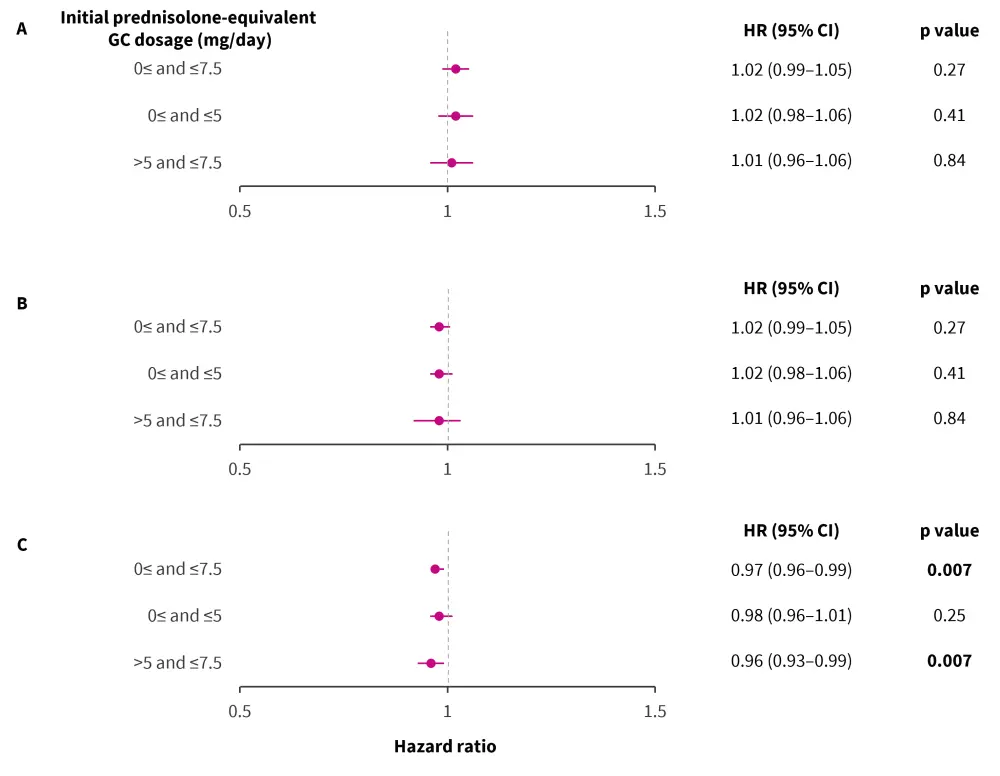
CI, confidence interval; GC, glucocorticoid; HR, hazard ratio; mSACQ, modified serologically active clinically quiescent; SLE, systemic lupus erythematosus.
*Data from Katsumata, et al.1
Damage accrual
- Of 1,677 mSACQ patients with available SDI data, 180 patients experienced new damage accrual.
- Tapering GCs was associated with a 4% decreased risk of damage accrual in the patients whose initial prednisolone dosages were 0–7.5 mg/day and >5 mg/day (Figure 1C).
- Use of antimalarials showed no association with damage accrual (HR, 1.10; 95% CI, 0.79–1.53; p = 0.59).
|
Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

