All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
The TULIP extension trial of anifrolumab in SLE: 3-year results
At the American College of Rheumatology (ACR) Convergence 2022, Kalunian1 presented results from the 3-year extension phase of the TULIP trials. The TULIP-1 and -2 phase III trials investigated anifrolumab, a human IgG1κ monoclonal antibody that inhibits type 1 IFN signaling, which is approved by the U.S. Food and Drug Administration (FDA) for the treatment of systemic lupus erythematosus (SLE).2 In both trials, patients were randomized to anifrolumab 300 mg, 150 mg, or placebo.1
The Lupus Hub has previously produced a visual abstract focused on glucocorticoid tapering in the TULIP studies. The extension phase of TULIP (NCT02794285) is the first long-term placebo-controlled safety study of anifrolumab.1
Patient disposition1
During the 3-year extension, patients received either anifrolumab 300 mg or placebo every 4 weeks, in addition to standard of care treatment. Patient disposition is shown in Figure 1. Fewer patients treated with placebo in the long-term extension completed this phase (48.2%) compared with patients treated with anifrolumab in the long-term extension, who had received either anifrolumab 300 mg (69.3%), anifrolumab 150 mg (61.2%), or placebo (62.2%) in the initial TULIP studies.
Figure 1. Patient disposition in the TULIP studies and extension*
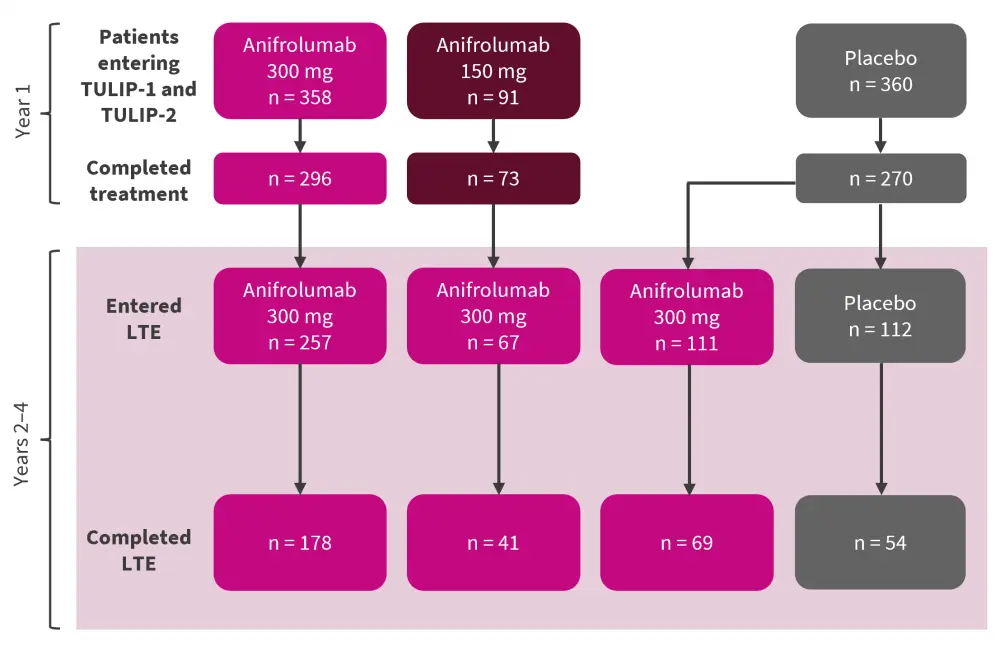
LTE, long-term extension.
*Adapted from Kalunian.1
Safety results1
Safety results for patients who received either anifrolumab 300 mg or placebo in TULIP-1 or -2 and remained on the same treatment in the long-term extension are shown in Table 1.
Table 1. Incidence of adverse events*
|
AE, adverse event; EAIR, exposure-adjusted incidence rate; MACE, major adverse cardiovascular event. |
||
|
AE, EAIR per 100 patient-years (unless otherwise stated) |
Anifrolumab 300 mg |
Placebo |
|---|---|---|
|
Exposure, years |
683.5 |
250.3 |
|
Any AE |
33.1 |
37.6 |
|
Any serious AE |
8.5 |
11.2 |
|
Any AE leading to death |
0.4 |
0.4 |
|
Any AE leading to treatment discontinuation |
2.5 |
3.2 |
|
Non-opportunistic serious infections |
3.7 |
3.6 |
|
Opportunistic infections |
0 |
1.2 |
|
Herpes zoster infection |
3.4 |
2.8 |
|
Latent tuberculosis |
2.3 |
0.8 |
|
Influenza |
2.2 |
0.8 |
|
MACE |
0.7 |
1.2 |
|
Malignancy |
0.3 |
0.8 |
|
Most common AEs |
|
|
|
Nasopharyngitis |
9.7 |
5.5 |
|
Urinary tract infection |
8.5 |
6.3 |
|
Upper respiratory tract infection |
8.3 |
7.2 |
Overall, 12 deaths occurred in the TULIP studies and long-term extension, nine of which were during the extension. Ten deaths were in patients treated with anifrolumab and two were in patients treated with placebo.
COVID-19 safety
Study drug exposure was higher in patients who received anifrolumab during the COVID-19 pandemic (227.7 patient-years; n = 325) than those who received placebo (42.7 patient-years; n = 64).
COVID-19-related adverse events were reported in 10.2% of patients who received anifrolumab and 6.3% of patients who received placebo. Serious COVID-19-related adverse events occurred in 4.9% and 1.6% of patients in the anifrolumab and placebo groups, respectively. Additionally, following adjustment for exposure and time-at-risk during the pandemic, rates of COVID-19-related serious adverse events were higher in patients treated with anifrolumab compared with placebo. However, no COVID-19-related events occurred following full vaccination, and all three deaths due to COVID-19 occurred during the first 6 months of the pandemic.
Efficacy results1
In patients who were treated with either anifrolumab 300 mg or placebo in the TULIP trials and remained on the same treatment in the long-term extension, the mean improvement in the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) at Week 52 of the extension was greater in those receiving anifrolumab compared with placebo, with improvements sustained over time. In addition, the mean glucocorticoid dose was lower at the end of the long-term extension for patients treated with anifrolumab than placebo, with an increase in the number of patients not requiring glucocorticoids in the anifrolumab group.
Figure 2. Glucocorticoid doses from baseline to Year 4 in the TULIP trials and long-term extension*
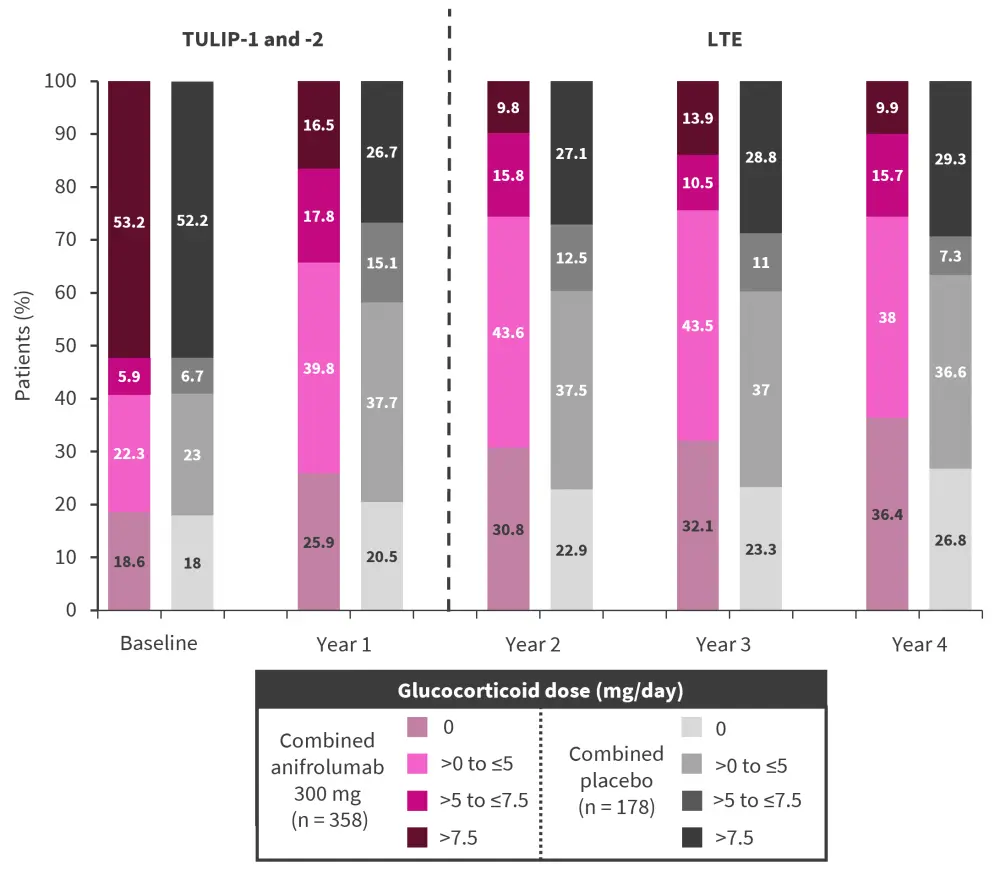
LTE, long-term extension
*Adapted from Kalunian.1
In each treatment year, fewer patients in the anifrolumab arm required the highest glucocorticoid dose (>7.5 mg/day) compared with placebo. Furthermore, the reduction from baseline to Year 4 in the proportion of patients treated with the highest glucocorticoid dose was greater in the anifrolumab arm than in the placebo arm.
Conclusion
This 3-year extension showed that anifrolumab is safe and effective when used long-term for the treatment of SLE. Rates of serious adverse events and serious infections were similar between the anifrolumab and placebo groups. However, rates of COVID-19-related serious adverse events were higher in patients treated with anifrolumab than placebo, highlighting the need for further studies into the safety of using anifrolumab in patients with COVID-19. Anifrolumab showed good efficacy in the long-term extension, with a reduction in the number of patients requiring high-dose glucocorticoids (>7.5 mg/day) and greater mean improvements in SLEDAI-2K compared with placebo.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

