All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Zetomipzomib, a first-in-class immunoproteasome inhibitor, for the treatment of SLE and LN
Zetomipzomib, a first-in-class immunoproteasome inhibitor, was evaluated for tolerability, safety, and exploratory efficacy in patients with systemic lupus erythematosus (SLE) with/without concurrent lupus nephritis (LN) in the phase Ib/II MISSION study (NCT03393013).
Results from the phase II portion of the study were presented at the American Society of Nephrology (ASN) Kidney Week 2022 Annual Meeting in Orlando, FL.1
A total of 21 adult patients were included, eligible patients had a diagnosis of active LN (Class III or IV +/- Class V) with a 24-hour urine protein to creatinine ratio (UPCR) of ≥1.0 mg/mg, despite previous treatment with a corticosteroid and at least one immunosuppressive drug for 8 weeks or more. Patients received a subcutaneous 60 mg dose of zetomipzomib weekly for 24 weeks, with an initial dose of 30 mg, and were followed-up for 12-weeks to assess safety. The primary endpoint was the number of patients achieving ≥50% reduction in UPCR from baseline after treatment (overall renal response; ORR). Safety was measured by incidence of treatment-emergent adverse events (TEAEs).
Results
Of the 21 patients who entered the study, 4 discontinued before the end of treatment. Efficacy was evaluated in the remaining 17 patients who completed treatment. The majority of the patients were female (90.5%) and the mean duration of SLE and LN was 9.7 years and 5.3 years, respectively. The most common TEAE was injection site reaction, experienced by 71.4% of patients, with serious TEAEs occurred in 2 (9.5%) patients (Table 1).
Table 1. Incidence of adverse events*
|
TEAE, treatment-emergent adverse event. |
|
|
Adverse event, % |
N = 21 |
|---|---|
|
Injection site reaction |
71.4 |
|
TEAE leading to discontinuation |
19.0 |
|
Grade 3 TEAE |
28.6 |
|
Serious TEAE |
9.5 |
|
Opportunistic infection |
0 |
|
Death |
0 |
Overall renal response up to Week 37 is shown in Figure 1.
Figure 1. Overall renal response with zetomipzomib treatment*
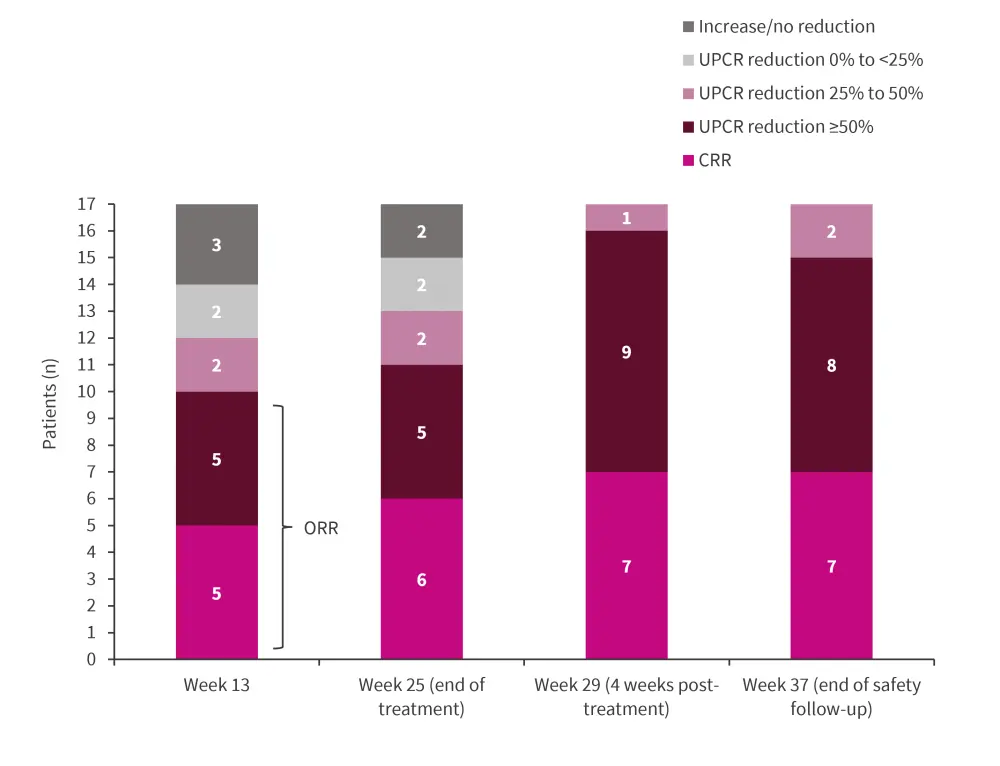
CRR, complete renal response; ORR, overall renal response; UPCR, urine protein to creatinine ratios.
*Adapted from Parikh, et al.1
The median UPCR from baseline to Week 37 is shown in Figure 2.
Figure 2. UPCR from baseline to Week 37*
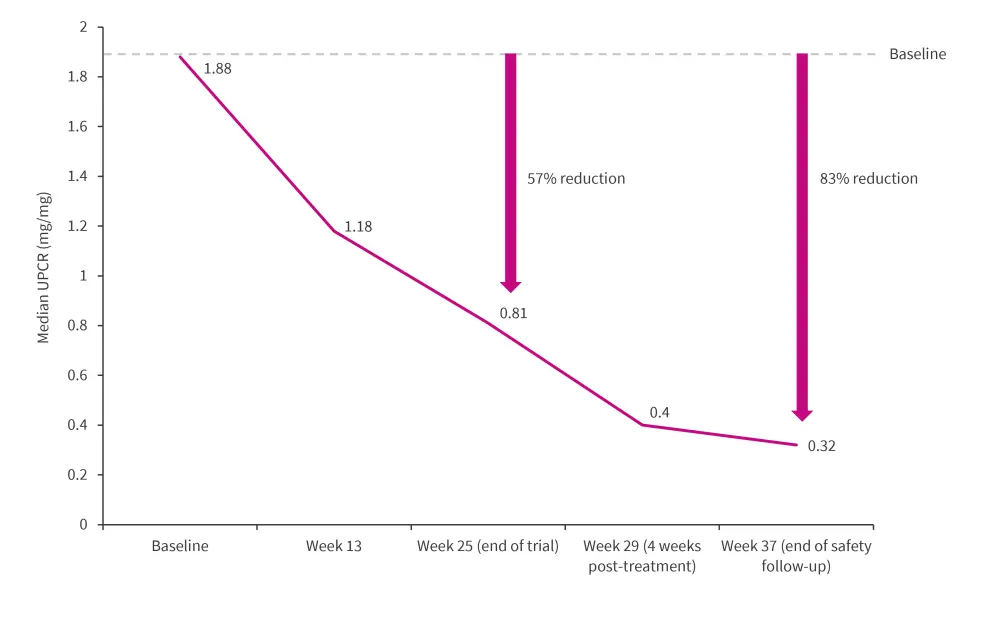
UPCR, urine protein to creatinine ratios.
*Adapted from Parikh, et al.1
ORR at Week 25 varied across LN biopsy classes. The overall ORR was 64.7%, with 60%, 70%, and 50% of patients with pure Class III, pure Class IV, and Class III/IV + Class V achieving ORR, respectively.
Other efficacy results showed that:
- Estimated glomerular filtration rate was stable through treatment and up to Week 37
- At Week 13, 82.4% of patients had achieved a daily corticosteroid dose of ≤10 mg
- The mean Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) reduced from 11.3 at baseline to 5.8 at Week 37
Conclusion
The authors highlighted that zetomipzomib may be an effective treatment add-on for patients with SLE and LN, with a favorable safety profile; 64.7% of patients achieved a reduction in UPCR of ≥50%, with many patients reducing their dose of corticosteroid.1 Renal responses remained consistent, regardless of LN class.1 However, the patient numbers in this study were low, additional studies with larger cohorts are planned to provide further efficacy and safety information in these patients.2
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

