All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Editorial theme | Agreement between 2021 Definition of Remission In SLE (DORIS) and physician-judged remission
The main target in the treatment of systemic lupus erythematosus (SLE) is to achieve remission.1 As previously published on the Lupus Hub, a treat-to-target (T2T) strategy in patients with SLE has the potential to improve short- and long-term outcomes such as a reduction in damage accrual, improvement in number of flares, and patients’ health-related quality of life.1
However, while applying a T2T strategy in clinical care, a standardized measure should be used to achieve a consistent management of SLE, rather than one based on physicians’ judgement alone. For measurement of disease activity, the Asia-Pacific Lupus Collaboration (APLC) has proposed Lupus Low Disease Activity State (LLDAS) as a treatment goal.1 For measurement of remission, an expert task force recently developed the 2021 Definition of Remission In SLE (DORIS). The definitions of DORIS and LLDAS can be found in the previously published article on the Lupus Hub.
To validate the expert-driven DORIS criteria in a clinical setting, González et al.1 recently published an article in Rheumatology that evaluated the agreement between the rate of 2021 DORIS remission and physician-judged remission in a large multicenter cohort of patients with SLE. Herein, we summarize the key findings.
Methods1
González et al. performed a cross-sectional analysis of data collected from a Spanish prospective, multicenter study involving patients with SLE in a clinical setting. Data collection was initiated in December 2018 and is ongoing. Experienced rheumatologists from seven Spanish rheumatology departments in tertiary university hospitals participated in the study.
Patients aged ≥18 years, meeting the revised 1997 American College of Rheumatology (ACR) classification criteria or the 2012 Systemic Lupus International Collaborating Clinics (SLICC) classification criteria for SLE were eligible.
As per the 2021 DORIS framework, the patients were classified according to several different definitions of remission: clinical remission, complete remission, clinical remission on treatment (ROT), and complete ROT.
Based on the assessments and clinical judgement, the rheumatologists were asked to categorize their patients into five different clinical states: remission, serologically active clinically quiescent (SACQ), or low, moderate or high disease activity. These categories were then compared with the DORIS definition of remission, using percentage of agreement and Cohen’s kappa as a measurement of agreement.
Results1
A total of 508 patients were included, of which 92% were women. The mean (standard deviation [SD]) age at diagnosis and enrolment was 40.7 (21.0) years and 50.4 (13.7) years, respectively, while the mean (SD) disease duration at enrolment was 10.8 (9.9) years. The baseline SLE criteria, SLE clinical variables, disease activity, and treatment at the time of enrolment are presented in Table 1.
Table 1. Baseline disease characteristics*
|
ACR, American College of Rheumatology; dsDNA, double-stranded DNA; SD, standard deviation; SLEDAI, SLE disease activity index; SLICC, Systemic Lupus International Collaborating Clinics; SLICC/ACR-DI, SLICC/ACR Damage Index. |
|
|
Characteristic, % (unless otherwise stated) |
All patients |
|---|---|
|
2012 SLICC criteria |
|
|
Arthritis |
69.9 |
|
Acute cutaneous lupus |
51.8 |
|
Leukopenia |
43.1 |
|
Oral or nasal ulcers |
33.7 |
|
Non-scaring alopecia |
30.7 |
|
Renal disorder |
31.1 |
|
Serositis |
18.9 |
|
Thrombocytopenia |
17.3 |
|
Immunological criteria |
|
|
Antinuclear antibody |
96.3 |
|
Anti-dsDNA antibodies |
64.8 |
|
Anti-Sm antibodies |
18.7 |
|
Low complement levels |
60.2 |
|
Anti-phospholipid antibodies |
32.1 |
|
Other characteristics at enrollment |
|
|
Mean number of ACR criteria for SLE (SD) |
5 (1.5) |
|
Mean number of SLICC criteria for SLE (SD) |
6.24 (2.2) |
|
Mean SLEDAI-2K score (SD) |
2.8 (3.3) |
|
Mean SLICC/ACR-DI score (SD) |
0.96 (1.4) |
|
Damage present |
49.8 |
|
Mean Physician Global Assessment score (SD) |
0.2 (0.49) |
|
Treatment at the time of enrolment |
|
|
Antimalarial |
74 |
|
Prednisone |
39 |
|
Immunosuppressant |
44 |
As per the 2021 DORIS, 54.4% of the patients achieved remission. Other states of remissions are presented in Table 2.
Table 2. Rates of remission as per 2021 DORIS*
|
DORIS, Definition of Remission In SLE; LLDAS, Lupus Low Disease Activity State; ROT, remission on treatment; SLE, systemic lupus erythematosus. |
|
|
Rate of remission, % |
All patients |
|---|---|
|
DORIS remission |
54.4 |
|
Clinical remission |
27.3 |
|
Complete remission |
24.4 |
|
Complete ROT |
46.4 |
|
LLDAS |
62.7 |
On the other hand, as per physicians’ assessment and judgement, 55.9% of the patients were classified as in remission (41.6%) or SACQ (14.3%), as shown in Figure 1.
Figure 1. Physician-judged SLE disease activity*
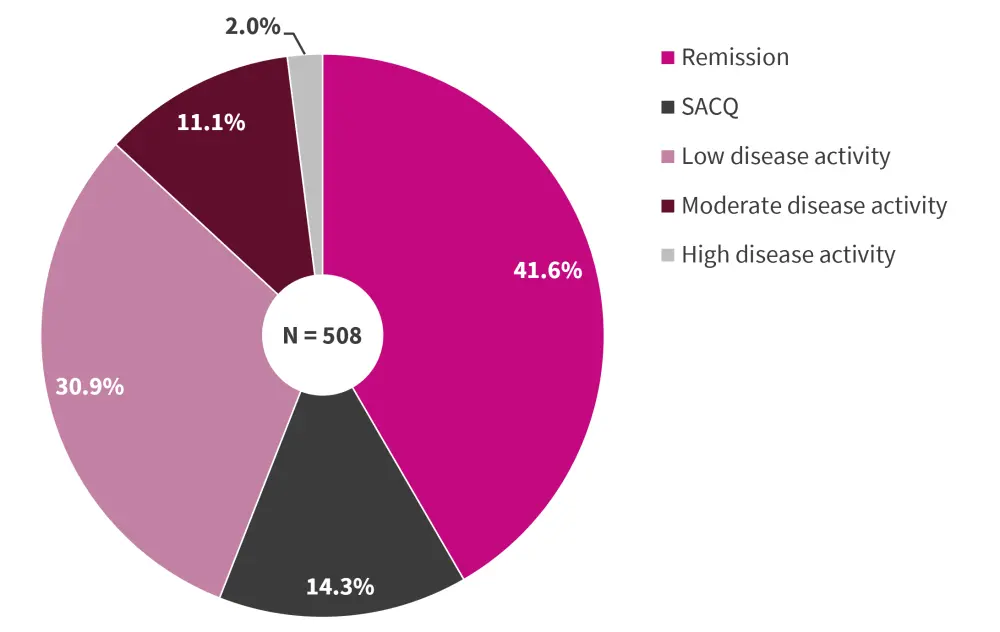
SACQ, serologically active clinically quiescent; SLE, systemic lupus erythematosus.
*Data from González, et al.1
The overall agreement between the 2021 DORIS remission and physician-judged remission/SACQ was 81.2% (95% confidence interval, 79.9–82.9%) and Cohen’s kappa agreement was 0.62 (0.55–0.69). Of 267 patients in remission as per the 2021 DORIS criteria, 83.1% patients were classified as in remission/SACQ by their physician (Figure 2).
Figure 2. Agreement between DORIS remission and physician-judged remission/SACQ*
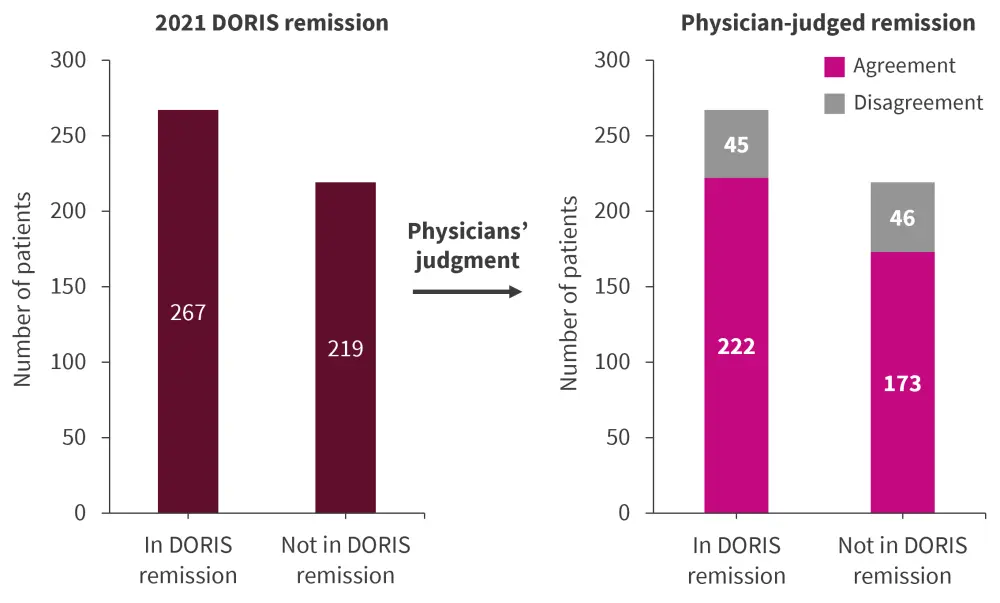
DORIS, Definition of Remission In SLE; SACQ, serologically active clinically quiescent.
*Data from González, et al.1
Among 219 patients not meeting the DORIS remission criteria, 78.9% patients were classified as not in state of remission by their physician (Figure 2). The reason for disagreement in 46 patients was that 39 (84.5%) patients did not meet the criterion of Clinical SLE Disease Activity Index (cSLEDAI) = 0 (median [IR] cSLEDAI was 3 [1–8]). The most common manifestations in these patients are shown in Figure 3.
Figure 3. Manifestations in patients meeting SLEDAI criteria of remission/SACQ by their rheumatologist but who did not meet 2021 DORIS remission criteria*
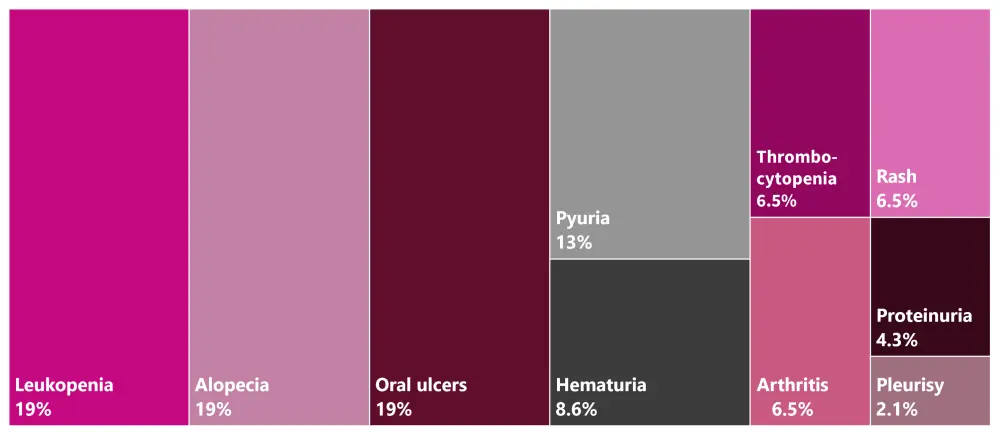
DORIS, Definition of Remission In SLE; SACQ, serologically active clinically quiescent; SLEDAI, Systemic Lupus Erythematosus Disease Activity.
*Data from González, et al.1
Conclusion1
The authors concluded that the 2021 DORIS is an achievable goal in the management of patients with SLE in clinical practice. There was a substantial agreement between the 2021 DORIS and physician-judged remission or SACQ. The main reason for disagreement was in patients who did not achieve remission as per DORIS but were classified as in remission by their physician was cSLEDAI score of >0, mainly due to ongoing mild activity.
A limitation of the study was the cross-sectional nature of the data from a single visit. Future longitudinal analysis will assess the relationship between these states of remission and LLDAS with long-term outcomes.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


