All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Efficacy and safety of gut microbiota-based therapies for SLE and LN
Patients with systemic lupus erythematosus (SLE) experience an imbalance in their intestinal flora. The use of probiotics as an adjunctive therapy may be considered to prevent vascular complications associated with SLE. Current evidence indicates the potential effectiveness of gut microbiota-based therapies for treating SLE and lupus nephritis (LN); however, a systematic summary is lacking.
Zeng et al.1 recently published an article in BMC Medicine reporting a systematic review and meta-analysis of randomized clinical trials assessing the efficacy and safety of probiotics in the treatment of various autoimmune diseases. Here, we summarize the findings for SLE and LN.
Methods1
-
A systematic literature search was performed across multiple databases to identify studies from inception to June 2022.
-
Outcomes were efficacy indicators (SLE Disease Activity Index [SLEDAI]), inflammatory factor indicators (Complement C3 and immunoglobulin G [IgG]), and adverse events.
-
Weighted mean differences were utilized to assess continuous variables with uniform measurement units.
Key findings
-
A total of four studies were included in the analysis for SLE and LN (Figure 1).1
Figure 1. Study characteristics*
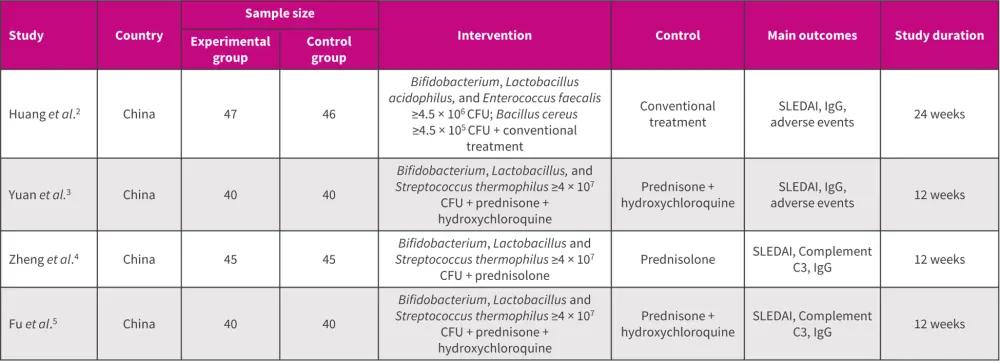
CFU, colony forming unit; IgG, immunoglobulin G; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index.
*Adapted from Zeng, et al.1
SLEDAI
-
Post gut microbiota-based therapy, SLEDAI was significantly lower in the experimental group compared with control group (p < 0.00001; Figure 2A).1
Complement C3
-
Blood complement C3 levels after gut microbiota-based therapy were significantly higher in the experimental group vs control group (p < 0.05).4,5
IgG level
-
Compared with control group, the IgG level in the experimental group was significantly lower after probiotic therapy (p < 0.00001; Figure 2B).1
Figure 2. A SLEDAI and B IgG level in patients with SLE and LN after gut microbiota-based therapy*
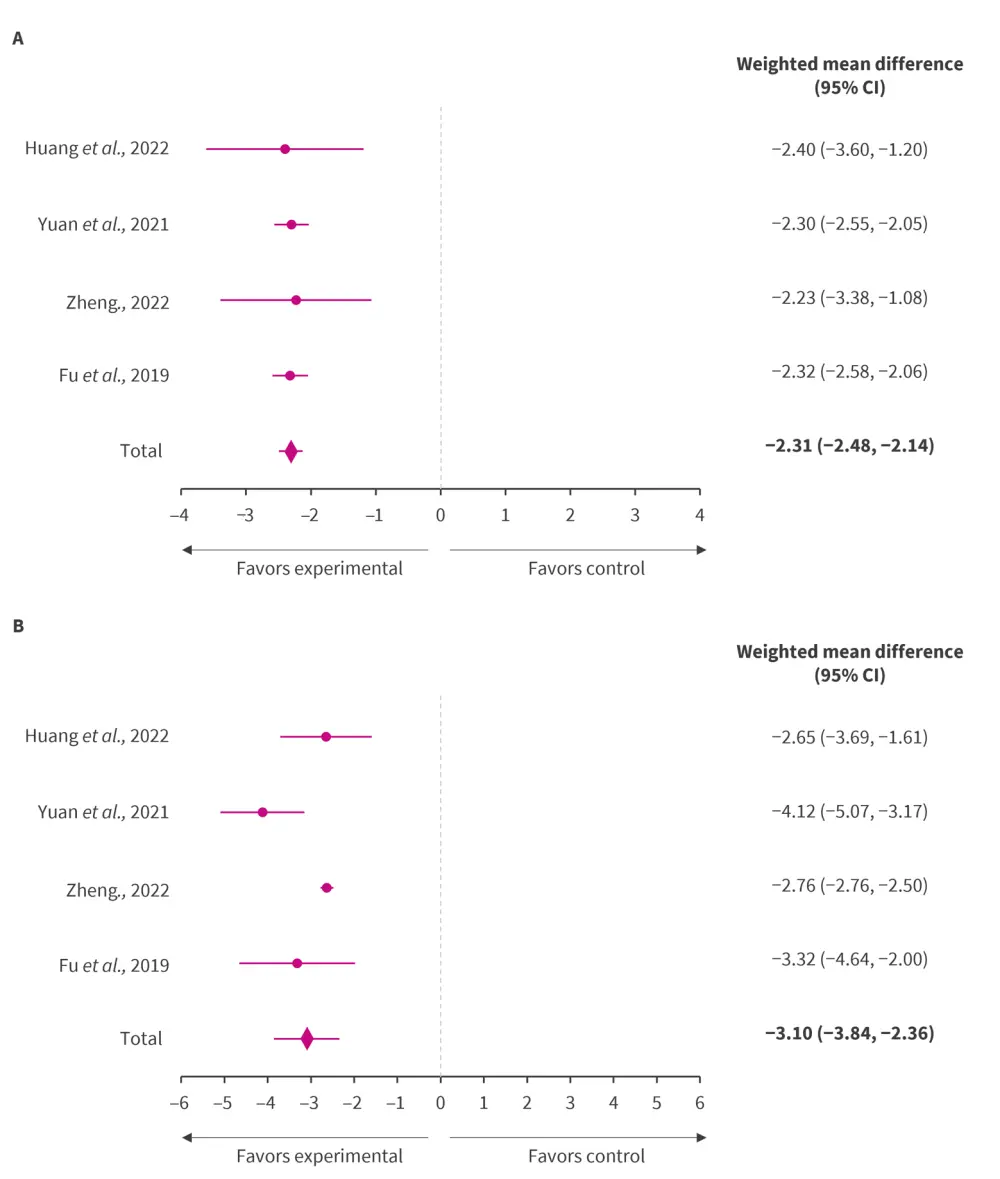
CI, confidence interval; IgG, immunoglobulin G; LN, lupus nephritis; SLE, systemic lupus erythematosus; SLEDAI, SLE Disease Activity Index.
*Adapted from Zeng, et al.1
Adverse events
-
A study by Huang et al. showed that the incidence of abnormal liver function, diarrhea, infection (upper respiratory tract, lung, and urinary tract), tachycardia, and other adverse events was not significantly different between the experimental group (31.91%) and control group (34.78%).2
-
Yuan et al. reported that no treatment-associated adverse events were reported.3
|
Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

