All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Factors underlying higher prevalence of systemic lupus erythematosus in females
Lupus predominantly affects women, with a 9:1 female-to-male ratio. Genetically, females carry XX genotypes, while males carry XY genotypes. To balance gene expression, every cell in a female silences one of the two X chromosomes using a long non-coding RNA (lncRNA) Xist. However, the genetic risk underlying autoimmune diseases from the second X chromosome remains unclear.
Dou et al.1 published an article in Cell investigating the role of Xist ribonucleoproteins (RNP) in sex-bias autoimmunity. Here, we summarize the key findings.
Methods1
- To induce Xist expression in male mice, a TetOP-∆RepA-Xist [tgXist] transgenic mouse model was developed. Two genetic backgrounds utilized were autoimmune-resistant C57BL/6J and autoimmune-prone SJL/J.
- Doxycycline was given with drinking water to detect Xist expression.
- Wild type (WT) and tgXist mice were injected with pristane to chemically induce systemic lupus erythematosus (SLE). The experimental cohorts are illustrated in Figure 1.
- To test the immunogenicity of Xist in humans, de-identified sera from patients with SLE were obtained.
Key findings1
- Female-biased autoimmunity: Bibliomic analysis found 30 proteins in Xist RNP that are targeted by autoantibodies in various human diseases.
- No disease activity in the genetically autoimmune resistant background: Pristane-treated tgXist male mice in nonpermissive C57/BL6 background did not manifest disease even after a year of treatment (contrasting the 16-week severe disease onset in the SJL/J background) and have low propensity to develop autoantibodies.
- Female-like changes in T-cell profiles: ATAC sequencing found elevated toll-like receptor 9 in tgXist-expressing males and Xist-expressing females. CIBERSORT analysis showed tgXist- or Cist-expressing males and WT females had more CD4 memory T cells, while control males had higher levels of naïve T cells.
- Multi-organ autoimmune pathology: Pristane treatment in tgXist males and WT females in autoimmune-prone SJL/J mouse background showed greater incidence and severity of glomerulonephritis, hepatic lipogranulomas, pulmonary hemorrhage and lymphohistiocytic alveolitis, compared with controls (Figure 1)
- Pristane-treated WT females showed elevated levels of four known autoantibodies (RIBO P0, RIBO P2, CENPA, and CENPB) compared with pristane-treated WT males. Pristane-treated tgXist males showed significant elevation in CENPB (p = 0.02) compared with pristane-treated WT males.
Figure 1. Increased pathophysiology in tgXist- and Xist-expressing mice in the SJL/J strain of the pristane-induced SLE model*
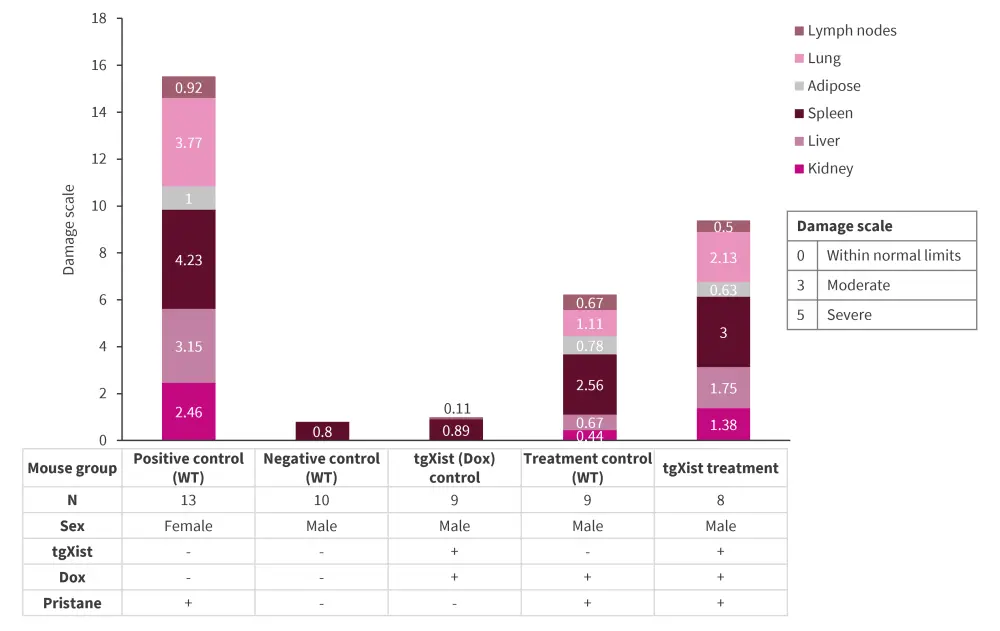
Dox, doxycycline; SLE, systemic lupus erythematosus; tgXist, TetOP-∆RepA-Xist; WT, wild type.
*Data from Dou, et al.1
- Higher autoimmunity: Compared with WT males, the tgXist-diseased males and WT females exhibited:
-
- upregulation of atypical B cell markers (Zeb2, FcrI5), Cd19, Ms4a1, and CD22; and
- downregulation of Cr2, Cr1I, Cd27, Cxcr5, key T-cell regulation genes (Nr3c1, Cd37, Cd52 Cd74), and self-tolerance genes (Siglec-g).
- Shared autoantibodies with autoimmune patients: Multiple proteins from the Xist RNP are novel autoantigens in patients with SLE, for example, HMGB1.
- Further, the female pristane-induced WT mice induced multiple autoantibodies to Xist RNP, that significantly overlapped with autoantibodies to Xist RNP in human patients with SLE (p = 0.001).
|
Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


