All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Ianalumab in patients with systemic lupus erythematosus: Interim results of a phase II trial
Ianalumab (VAY736), an afucosylated human immunoglobulin G1 monoclonal antibody targeting the B-cell activating factor receptor (BAFFR), is a novel therapeutic agent for systemic lupus erythematosus (SLE) which depletes B cells through enhanced antibody-dependent cellular cytotoxicity and concurrent BAFF:BAFFR blockade.1,2
Below, we summarize the interim findings from a phase II trial (NCT03656562) evaluating ianalumab in patients with SLE, presented by Shen et al.1 and Santos da Costa et al.2 at the American College of Rheumatology (ACR) annual meeting (ACR Convergence 2023).
Study design
This was a multicenter, randomized, double-blind placebo-controlled trial. The study design is depicted in Figure 1.
Figure 1. Study design*
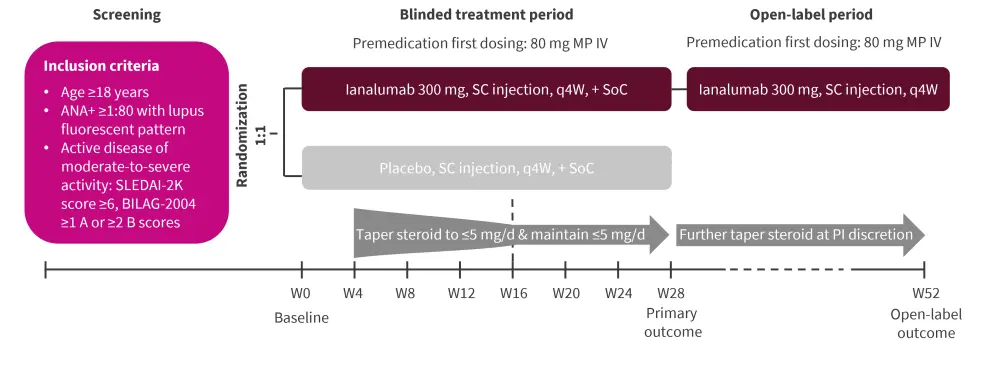
ANA, antinuclear antibody; BILAG, British Isles Lupus Assessment Group; d, day; IV, intravenous; MP, methylprednisolone; PI, principal investigator; q4W, every four weeks; SC, subcutaneous; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000; SoC, standard of care; W, week.
*Adapted from Shen, et al.1
†Or <baseline, whichever is lower.
Key findings1,2
In total, 34 patients (median age, 42 years) were treated with ianalumab and 33 (median age, 39 years) were treated with placebo; 94.1% and 81.8% were female, respectively.1
Treatment with ianalumab resulted in:
- higher proportion of patients achieving SLE Responder Index-4 response with sustained steroid reduction (primary composite endpoint) and Lupus Low Disease Activity State, and reduced incidence of moderate or severe flare (Figure 2A).1
- substantial and significant reduction in total B cells, including naïve and memory B cells, and antibody-producing subsets, while preserving levels of CD4+ and CD8+ T cells or natural killer cells.2
- strong reduction in B-cell-related genes, a trend for downregulation of interferon gene signature,2 and an acceptable safety profile (Figure 2B).1
Figure 2. A Efficacy and B safety of ianalumab*
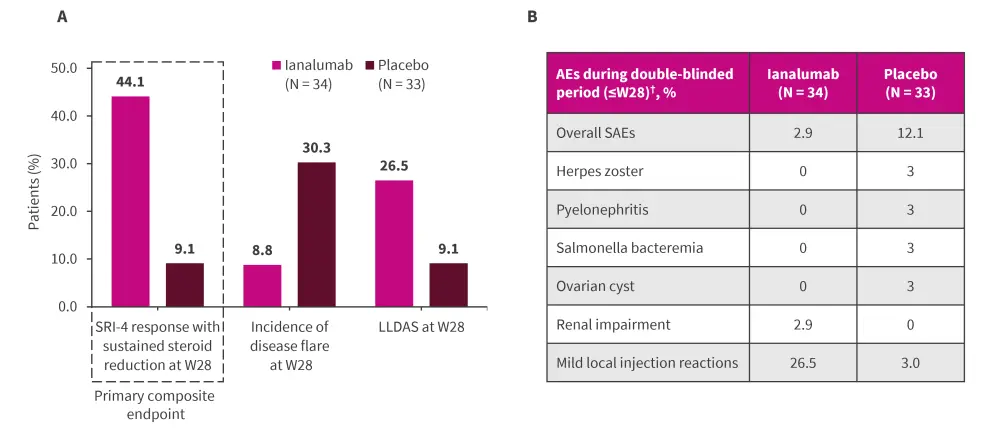
AE, adverse event; LLDAS, Lupus Low Disease Activity State; SAE, serious AE; SRI-4, Systemic Lupus Erythematosus Responder Index-4; W, week.
*Adapted from Shen et al.1
†All events occurred before the first administration of open-label treatment.
|
Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


