All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Impact of belimumab on renal and neuropsychiatric flares in patients with SLE and LN
Systemic lupus erythematosus (SLE) is a heterogeneous immune-mediated disorder that can affect multiple organs and tissues.1–3 SLE impacts the kidneys in up to 60% of patients, with up to 30% of those patients progressing to end-stage kidney disease, making lupus nephritis (LN) one of the major causes of morbidity and mortality in SLE.1,2
Studies have also revealed involvement of the nervous system in around 30–40% of patients.3 Manifestations of neuropsychiatric (NP) SLE range from mild symptoms, such as headache and subtle cognitive impairment, to severe or potentially life-threatening events including psychosis, seizures, and cerebrovascular accidents. These manifestations substantially contribute to organ damage and a decline in health-related quality of life.3
Therefore, prevention of renal and NP flares is a key component of effective disease management and can improve overall outcome in patients with lupus. Belimumab is the first human monoclonal antibody approved as an add-on treatment for adults with active SLE and LN.2 It has demonstrated an ability to control disease and prevent SLE flares in multiple clinical trials.2 In this article, we provide an overview of recently reported benefits of belimumab in the prevention of renal and NP flares in patients with SLE and LN.
Renal flares in patients with LN receiving belimumab versus control1
Zhang et al.1 recently published a systematic review and meta-analysis of belimumab in patients with LN in Renal Failure. Of the six randomized clinical trials (RCTs) included in the analysis, four had data on renal flare with 65 weeks of mean belimumab treatment time. The key characteristics and renal flare outcomes of these studies are presented in Table 1.
Table 1. Characteristics and outcomes of studies included in the meta-analysis*
|
HR, hazard ratio; CI, confidence interval; RR, risk ratio; SD, standard deviation. |
||||
|
Study |
Patients |
Mean age ± SD, years |
Outcomes |
|
|---|---|---|---|---|
|
Belimumab |
Control |
|||
|
BLISS-52 (NCT00424476) and BLISS-76 (NCT00410384)4 |
N = 1,125 |
38.0 ± 11.0 |
38.1 ± 11.9 |
Patients treated with belimumab 10 mg/kg experience numerically lower renal flare rate (1.4%) compared with belimumab 1 mg/kg (2.5%) or placebo (3.0%). |
|
BLISS-SC (NCT01484496)5 |
N = 836 |
38.1 ± 12.10 |
39.6 ± 12.61 |
Fewer patients in the belimumab group had a renal flare compared with placebo (4.7% vs 7.5%; HR, 0.57; 95% CI, 0.32–1.01; p = 0.0532). |
|
BLISS-LN (NCT01639339)1 |
N = 448 |
33.7 ± 10.7 |
33.1 ± 10.6 |
Patients on belimumab had a significantly lower risk of renal flare than the control group (RR, 0.55; 95% CI, 0.37–0.84). |
|
EMBRACE (NCT01632241)6 |
N = 448 |
38.6 ± 11.1 |
39.3 ± 12.2 |
Patients receiving belimumab had a 46% lower risk of renal flare compared with those receiving placebo (HR, 0.54; 95% CI, 0.21–1.36; p = 0.1880). |
In the pooled analysis these studies, risk of renal flares was significantly lower in the belimumab group compared with the control group (Figure 1).1
Figure 1. Renal flares in belimumab compared with control groups*†‡
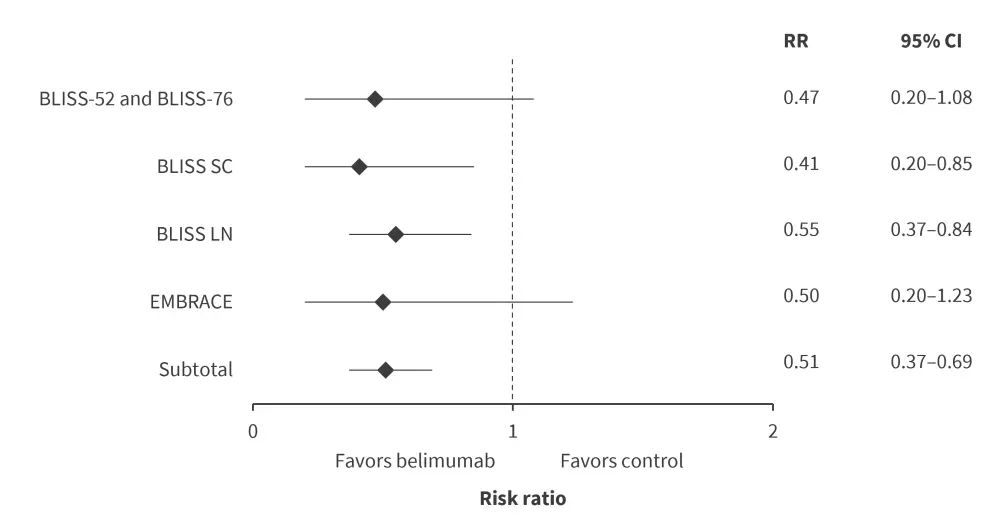
CI, confidence interval; RR, risk ratio.
*Adapted from Zhang, et al.1
†Heterogeneity: Chi2 = 0.54; df = 3 (p = 0.91); I2 = 0%.
‡Test for overall effect: Z = 4.27 (p < 0.0001).
De novo renal flares in patients with SLE receiving belimumab versus control7
Parodis et al.7 presented data at the European Alliance of Associations for Rheumatology (EULAR) 2023 Congress on the occurrence of de novo LN flares in patients with SLE with no prior history of renal disease undergoing standard therapy with add-on belimumab. The post-hoc analysis included the following five phase III RCTs:
- BLISS-52 (N = 865);
- BLISS-76 (N = 819);
- BLISS-NEA (NCT01345253; N = 677);
- BLISS-SC (N = 836); and
- EMBRACE (N = 448).
Patients were included with baseline renal British Isles Lupus Assessment Group (BILAG) score E. De novo renal flare was defined as a transition from BILAG E to A or B within a 52-weeks of follow-up.
The study revealed a significant reduction in the occurrence of de novo renal flare among patients treated with belimumab IV 1 mg/kg compared with those treated with placebo, whereas belimumab IV 10 mg/kg and belimumab SC 200 mg was not significantly different to placebo (Figure 2A).
In multivariable cox regression analysis, use of belimumab IV 1 mg/kg showed significantly decreased incidence of de novo renal flare, followed with belimumab IV 10 mg/kg, whereas belimumab SC 200 mg displayed no clear protection (Figure 2B).
Figure 2. Proportion of patients developing de novo renal flare and B multivariable Cox regression analysis showing hazard risk of renal flare*
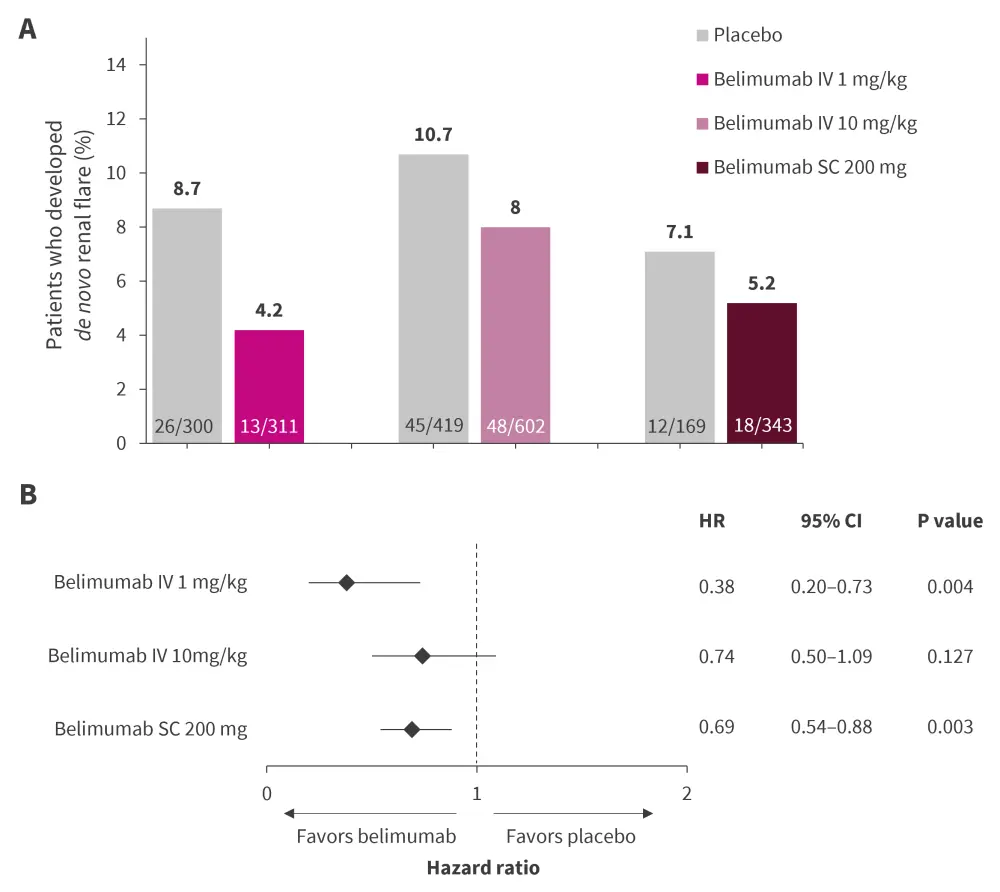
CI, confidence interval; HR, hazard ratio; IV, intravenous infusion; SC, subcutaneous.
*Adapted from Parodis, et al.7
Renal flares in patients with extra-renal SLE receiving belimumab and antimalarials2
A post-hoc analysis was performed by Gomez et al.2 to determine the effect of different doses of belimumab and antimalarial agents (AMA) for the prevention of renal flares in patients with moderately to highly active extra-renal SLE. Data was pooled from the following RCTs:
- BLISS-52 (N = 865);
- BLISS-76 (N = 819);
- BLISS-SC (N = 836); and
- BLISS-NEA (N = 705).
In multivariable Cox regression analysis, risk of renal flares was lower among patients receiving belimumab IV 1 mg/kg and 10 mg/kg, whereas no significant association was found for belimumab SC 200 mg (Figure 3). Notably, patients receiving IV belimumab 1 mg/kg along with AMA experienced the lowest rate of renal flares (18.5 cases), compared with those receiving IV belimumab 10 mg/kg + AMA (56.3 cases), belimumab SC 200 mg + AMA (45.4 cases) or placebo + AMA (81.7 cases) per 1,000 person years.
Figure 3. Combination effect of belimumab and AMA on renal flare prevention*
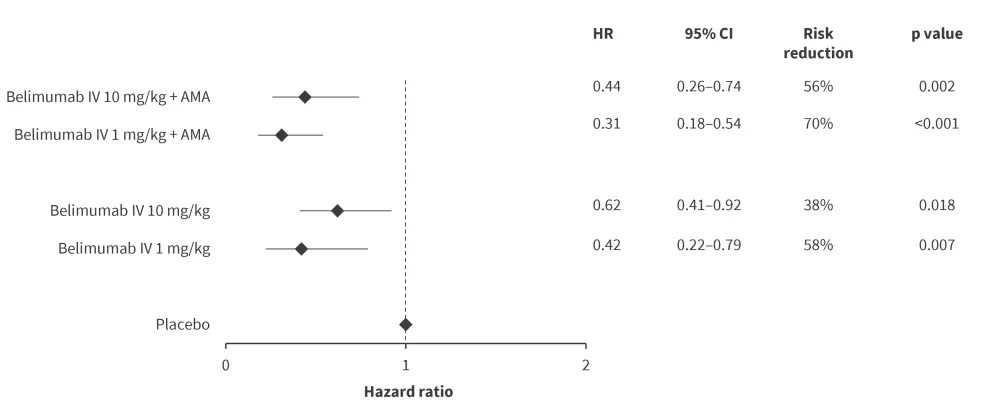
AMA, antimalarial agents; CI, confidence interval; HR, hazard ratio; IV, intravenous.
*Data from Gomez, et al.2
NP flares in patients with SLE receiving belimumab versus control3,8
At the EULAR 2023 Congress, Palazzo et al.8 presented another post-hoc analysis on the impact of belimumab on NP flares in patients with SLE. The study utilized the same five studies as the Parodis et al.7 study mentioned above.
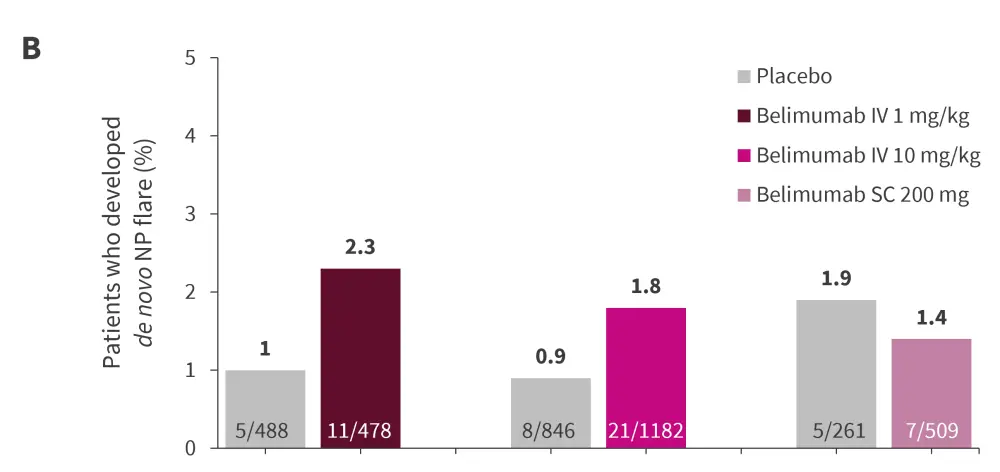
After 52 weeks of follow-up, there were no significant differences in occurrence of NP/SLE flare (Figure 4A) and de novo NP/SLE flare (Figure 4B) in patients treated with belimumab and those treated with placebo.
Figure 4. Proportion of patients developing A NP/SLE flare and B de novo NP/SLE flare*
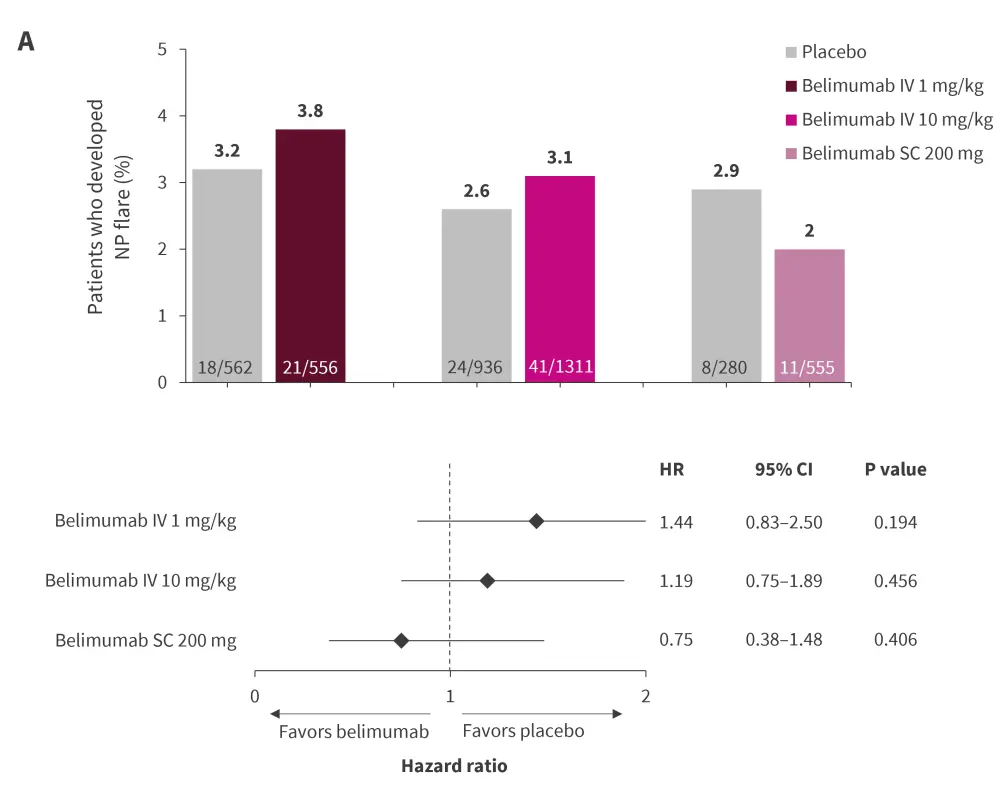
IV, intravenous infusion; NP, neuropsychiatric; SC, subcutaneous; SLE, systemic lupus erythematosus.
*Data from Palazzo, et al.3,8
Conclusion1-8
The meta-analysis found that belimumab had a significant impact in reducing risk of renal flare compared with control group. The post-hoc analyses further revealed that add-on low-dose IV belimumab 1 mg/kg on top of standard-therapy appeared protective against de novo renal flares, while the approved 10 mg/kg IV belimumab dose and SC belimumab 200 mg didn’t offer any clear protection. Additionally, the benefits conferred by belimumab IV 1 mg/kg seem to be enhanced when administered alongside antimalarials.
The potential benefits of belimumab treatment in preventing NP/SLE flares remains uncertain and further investigation in future studies is warranted.
The efficacy of lower doses of belimumab warrants exploration of the effectiveness of intermediate doses.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

