All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Impact of mycophenolate mofetil discontinuation in patients with SLE
Mycophenolate mofetil (MMF) is a commonly used immunosuppressant to treat systemic lupus erythematosus (SLE) and lupus nephritis. However, long-term treatment with MMF increases the risk of adverse events, including infections and malignancies, requiring withdrawal following disease quiescence. Furthermore, the risks of MMF withdrawal are unclear.
Chakravarty et al. published an article in The Lancet Rheumatology,1 assessing the risks associated with MMF discontinuation vs maintenance in patients with quiescent SLE on long-term MMF. Here, we summarize the key findings.
Methods1
- This was an open-label, multicenter, randomized controlled trial (NCT01946880).
- Patients were randomly allocated 1:1 to MMF maintenance and withdrawal groups.
- The primary endpoint was the probability of clinically significant disease reactivation by Week 60.
- To evaluate non-inferiority, an estimation-based approach was used to calculate absolute increases in risk associated with MMF withdrawal, with one-sided upper 75%, 85%, and 95% confidence limits.
Key findings1
- A total of 49 patients in the maintenance group and 51 patients in the withdrawal group were included in the modified intent-to-treat population.
- Mean age was 42 years, 84% of patients were women, average MMF treatment duration was 6.6 years, and 76% of patients had a history of lupus nephritis.
- MMF withdrawal led to a non-significant 7% increase in the risk of clinically significant disease reactivation by Week 60 (Figure 1A).
- Mean time to clinically significant disease reactivation was 38 weeks for the MMF maintenance group and 38.5 weeks for the MMF withdrawal group.
- Risk estimates for all other disease measures overlapped between maintenance and withdrawal groups (Figure 1A).
- In the subgroup of patients with renal disease, point estimates and one-sided confidence limits for increases in risk with MMF withdrawal were higher compared with the modified intention-to-treat group (Figure 1B).
Figure 1. Increase in risk with MMF withdrawal at Week 60 in A modified intent-to-treat population, and
B subset of patients with renal disease*
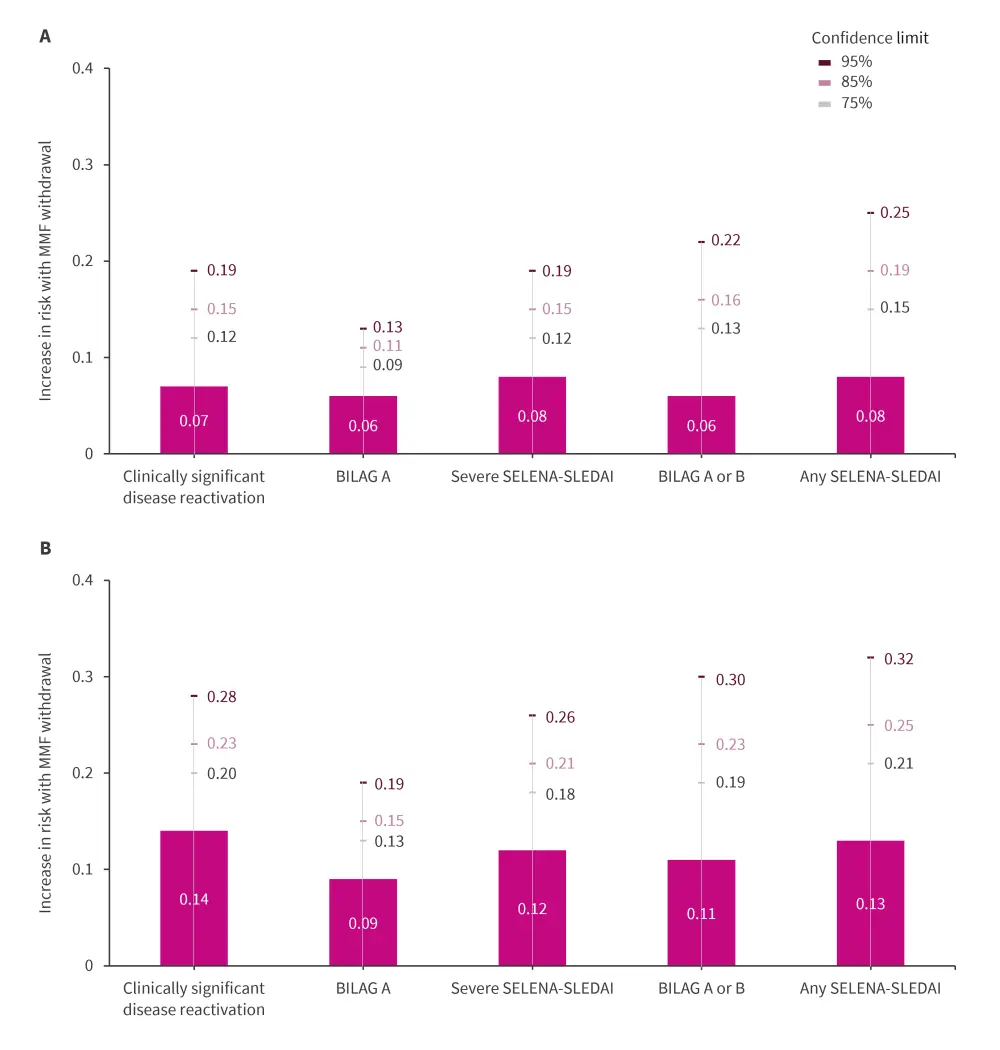
BILAG, British Isles Lupus Assessment Group; MMF, mycophenolate mofetil; SELENA-SLEDAI, Safety of Estrogens in Lupus Erythematosus National Assessment-Systemic Lupus Erythematosus Disease Activity Index.
*Data from Chakravarty, et al.1
Safety
- Adverse events were similar between groups; however, the MMF maintenance group reported more frequent infections and a shorter time to first infection compared with the withdrawal group (Table 1).
- One pregnancy happened nine months post complete MMF withdrawal, with a full-term healthy infant.
- No deaths were reported.
Table 1. AEs in the safety population*
|
AE, adverse event, SLE, systemic lupus erythematosus. |
||
|
Events, % |
Maintenance cohort |
Withdrawal cohort |
|---|---|---|
|
Serious AEs |
14 |
10 |
|
Total AEs |
90 |
88 |
|
Related to SLE |
50 |
62 |
|
AE by severity |
|
|
|
Grade 1 |
0 |
2 |
|
Grade 2 |
90 |
87 |
|
Grade 3 |
20 |
21 |
|
Grade 4 |
4 |
0 |
|
Total infections |
64 |
46 |
|
Infections by severity |
|
|
|
Grade 2 |
60 |
46 |
|
Grade 3 |
8 |
2 |
|
Grade 4 |
0 |
0 |
|
Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


