All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Management of systemic lupus erythematosus and lupus nephritis: Updated 2023 recommendations from EULAR
Do you know... According to the 2023 EULAR recommendations for management of SLE, what is the maximum daily dose of prednisone considered 'acceptable' for maintenance therapy?
The European Alliance of Associations for Rheumatology (EULAR) released its first recommendations for management of systemic lupus erythematosus (SLE) in 2008, followed by its update in 2019. Since then, rapid developments have occurred in SLE treatment paradigm, including a shift from traditional ‘induction-maintenance’ strategy to the early combination therapies. The non-pharmacological management strategies recommended by EULAR in 2023 have been previously described on the Lupus Hub.
Here, we provide an overview of the 2023 updates of the EULAR recommendations for the management of SLE, published by Fanouriakis, et al. in Annals of the Rheumatic Diseases.1 Additionally, we outline the 2022 EULAR recommendations for prevention of cardiovascular risk in patients with SLE, published by Drosos, et al. in Annals of the Rheumatic Diseases.2
Methods1
An international task force formed the research questions for the systematic literature reviews conducted between January 2018 and December 2022. The overarching principles and recommendations were developed and finalized through a predefined voting process during a series of meetings. Levels of evidence and strengths of recommendation were determined, and participants indicated their agreement with each item on a scale of 0 (no agreement) to 10 (100% agreement).
Results
Overarching principles1
Five overarching principles (Figure 1) and 13 recommendations (Figure 2) were formulated.
Figure 1. Overarching principles for management of SLE*

SD, standard deviation; SLE, systemic lupus erythematosus; QoL, quality of life.
*Adapted from Fanouriakis, et al.1
Figure 2. EULAR 2023 recommendations for management of patients with SLE*
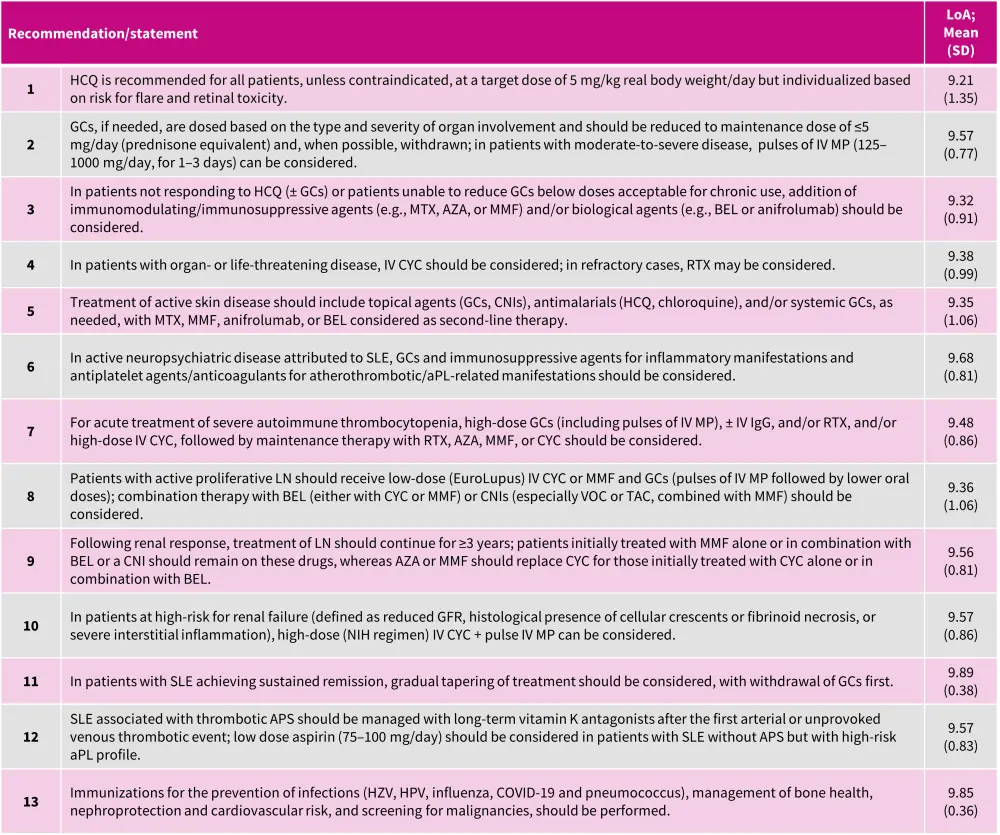
aPL, antiphospholipid antibodies; APS, antiphospholipid syndrome; AZA, azathioprine; BEL, belimumab; CNI, calcineurin inhibitor; COVID, Coronavirus disease; CYC, cyclophosphamide; GC, glucocorticoid; GFR, glomerular filtration rate; HCQ, hydroxychloroquine; HPV, human papillomavirus; HZV, herpes zoster virus; Ig, immunoglobulin; IV, intravenous; LoA, level of agreement; LN, lupus nephritis; MMF, mycophenolate mofetil; MP, methylprednisolone; MTX, methotrexate; NIH, National Institutes of Health; RTX, rituximab; SD, standard deviation; SLE, systemic lupus erythematosus; TAC, tacrolimus; VOC, voclosporin.
*Adapted from Fanouriakis, et al.1
The major updates since 2019 recommendation are briefly described below:
Recommendation 1
In moderate or severe cases, the initial hydroxychloroquine (HCQ) dose can exceed 5 mg/kg/day (up to 400 mg/day), later adjusted based on the patient's improvement. Additionally, it is suggested to monitor HCQ blood levels to guide optimal dose and assess treatment adherence.
In countries with limited access to HCQ, chloroquine may be considered as an alternate option; however, it is associated with a higher risk of retinal toxicity than HCQ.
Recommendation 2
Notably, the ‘acceptable’ daily prednisone dose for maintenance treatment has been reduced to a maximum of 5 mg/day prednisone equivalent, as compared with 7.5 mg/day in the 2019 recommendations.
Recommendation 3
This recommendation highlights the inclusion of anifrolumab in the treatment landscape, as a second biological agent approved in 2021. Notably, there is no hierarchy in the choice between anifrolumab and belimumab (BEL), and prior immunosuppressive treatment is not a requirement for starting a biologic.
Recommendation 4
Patients not responding to combination therapies (cyclophosphamide [CYC] + rituximab [RTX]) or sequential strategies (RTX followed by BEL) might consider alternative options like plasma exchange, hematopoietic stem cell transplantation, or chimeric antigen receptor T-cells; however, more long-term data are warranted.
Recommendation 5
Anifrolumab and BEL are both effective in mucocutaneous SLE. However, only anifrolumab utilized the Cutaneous Lupus Area and Severity Index in its clinical trials, while BEL assessed responses using the general instruments like Systemic Lupus Erythematosus Disease Activity Index and British Isles Lupus Assessment Group. Additional treatments like dapsone, retinoids, calcineurin inhibitors, azathioprine, CYC, and RTX, could be considered as second/third line options under the guidance of dermatologist. Thalidomide and lenalidomide should be reserved for patients not responding to prior treatments and should be used cautiously in women of childbearing age.
Recommendation 6
There have been no major development in the treatment of neuropsychiatric SLE since 2019, so the current recommendation aligns with the previous update. Patients with severe neuropsychiatric SLE are not recommended BEL or anifrolumab.
Recommendation 7
There have been no major advancements in the treatment of autoimmune thrombocytopenia in SLE since 2019, so the current recommendation remains consistent with the previous update.
Recommendation 8
Both BEL and voclosporin (VOC), are approved for all patients with active LN and can be used as first-line treatments. Based on the physician’s discretion, early combination therapy should be considered in all adult patients with active LN. In cases where treatment-naïve patients with LN do not opt for combination therapy, add-on treatment with BEL or VOC should be considered for those responding within 3–6 months, or those who flare.
Recommendation 9
Immunosuppressives in proliferative LN should be maintained for ≥3 years. Compared to mycophenolate mofetil/calcineurin inhibitors combination, the mycophenolate mofetil/ VOC combination showed stable glomerular filtration rate levels over 3 years in clinical trial.
Recommendation 10
For patients with impaired kidney function, the traditional high-dose intravenous CYC regimen (0.5–0.75 g/m2 monthly for 6 months) can also be considered.
Recommendation 11
For immunosuppressants, especially in LN cases, the duration of therapy and achieving remission are important; patients should undergo 3–5 years of therapy and maintain ≥2 years of remission before attempting withdrawal, following a gradual tapering process.
Unlike GCs and immunosuppressive drugs, patients with SLE should not withdraw HCQ unless there are unacceptable adverse events. HCQ provides multiple benefits, including increased survival and protection against disease relapse in patients discontinuing GCs or immunosuppressants.
Recommendation 12
HCQ, known for its antithrombotic effects and potential to reduce antiphospholipid antibodies (aPL) levels, is recommended for patients with SLE-aPL or SLE/antiphospholipid syndrome (APS). Management of patients with catastrophic APS involves triple therapy with full anticoagulation, high-dose GCs, and plasma exchange, and/or intravenous immunoglobulin. Eculizumab, a complement inhibitor, shows promise in catastrophic APS with complement-mediated thrombotic microangiopathy.
Recommendation 13
The Lupus Hub has previously published the 2019 EULAR recommendations for infection prevention and vaccination administration. The live attenuated and recombinant zoster vaccines are deemed safe, while SARS-CoV-2 vaccines are recommended in patients with SLE due to their proven immunogenicity and safety.
EULAR recommendations for treatment of extrarenal SLE and LN1
The treatment approach recommended by EULAR for the management of extrarenal SLE and LN is outlined in Figure 3.
Figure 3. Treatment of A. extrarenal SLE and B. LN*
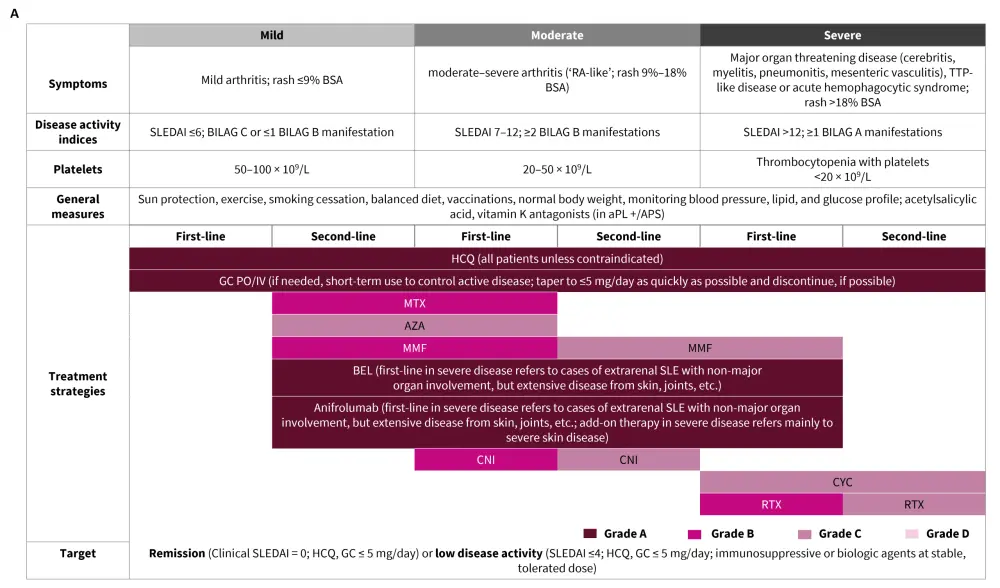
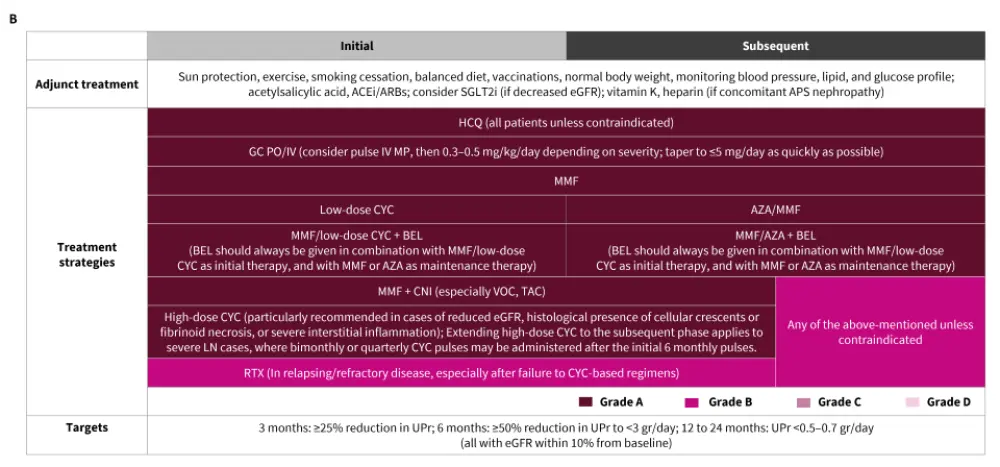
ACEi, angiotensin-converting enzyme inhibitors; aPL, antiphospholipid antibodies; APS, antiphospholipid syndrome; ARB, angiotensin receptor blockers; AZA, azathioprine; BEL, belimumab; BILAG, British Isles Lupus Assessment Group; CNI, calcineurin inhibitor; CYC, cyclophosphamide; eGFR, estimated glomerular filtration rate; GC, glucocorticoid; HCQ, hydroxychloroquine; IV, intravenous; MMF, mycophenolate mofetil; MP, methylprednisolone; MTX, methotrexate; PO, per os; RTX, rituximab; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; TAC, tacrolimus; UPr, urine protein; VOC, voclosporin.
*Adapted from Fanouriakis, et al.1
EULAR recommendations for the management of cardiovascular risk2
The recommendations for the management of cardiovascular risk are in line with the previously published guidelines by EULAR in 2022 (Figure 4).2
Figure 4. Management of cardiovascular risk in patients with SLE and aPL*
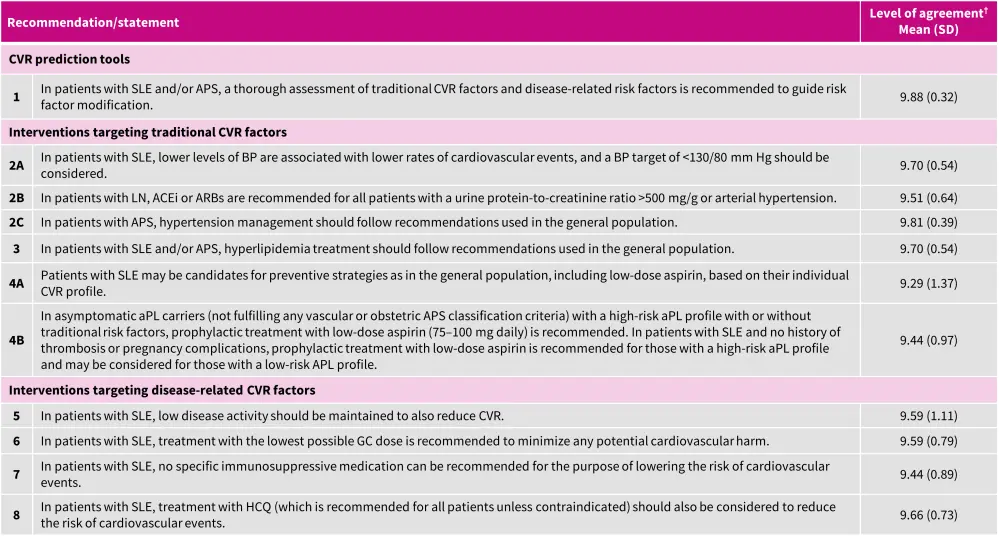
ACEi, angiotensin-converting enzyme inhibitor; aPL, antiphospholipid antibodies; APS, antiphospholipid syndrome; ARB, angiotensin II receptor blocker; CVR, cardiovascular risk; GC, glucocorticoid; HCQ, hydroxychloroquine; LN, lupus nephritis; SD, standard deviation; SLE, systemic lupus erythematosus.
*Adapted from Drosos, et al.2
Conclusion1
The 2023 EULAR recommendations for the management of SLE provide comprehensive guidance to healthcare professionals worldwide. Further quality indicators need to be developed and validated to ensure a wider dissemination and implementation of these guidelines in real-life clinical practice.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




