All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Long-term benefit of anifrolumab in SLE: post-hoc analyses of phase III TULIP-LTE
Here, we summarize post-hoc analyses of the TULIP-LTE trial presented at the American College of Rheumatology (ACR) annual meeting (ACR Convergence 2023).
Study design
Adults with moderate-to-severe SLE completing the 52-week phase III TULIP-1/TULIP-2 trials reconsented to enroll in the randomized, placebo-controlled, double-blind LTE trial.
From Week 52 through Week 208, the outcomes assessed were:
- overall and organ-specific Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) improvements1;
- SLEDAI-2K renal improvement2;
- patient-reported outcomes by the Short Form 36 Health Survey Version 23; and
- attainment of Definition of Remission in SLE (DORIS).4
Key findings
At Week 208, anifrolumab vs placebo was associated with a:
- higher proportion of patients achieving ≥4 point reduction in overall SLEDAI-2K (Figure 1) and individual organ domains1;
- higher proportion of patients achieving renal improvement, with or without SLEDAI-2K renal involvement at baseline (Figure 1)2;
- higher proportion of patients responding to physical and mental component scores (Figure 1), and Short Form 36 Health Survey Version 2 domains3; and
- shorter time to first DORIS remission (median 13.5 vs 15.0 months; p = 0.0338) and increased DORIS remission attainment (Figure 1).4
Figure 1. Post-hoc analyses of the phase III TULIP-LTE trial*
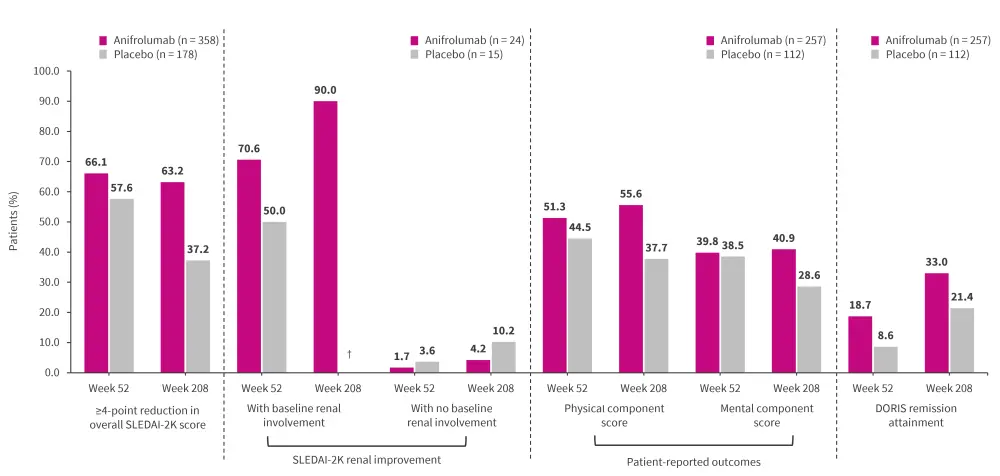
DORIS, Definition of Remission in SLE; LTE, long-term extension; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index.
*Data from Furie, et al.1; Furie, et al.2; Strand, et al.3; and van Vollenhoven, et al.4
†No placebo patients had available data at Week 208.
|
Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


