All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Post-hoc analyses of pooled data from five phase III trials of belimumab
Belimumab is approved for treating active systemic lupus erythematosus (SLE) and lupus nephritis, alongside standard therapy.
During the American College of Rheumatology annual meeting (ACR Convergence) 2023, Parodis et al.1 and Sheikh et al.2 presented post-hoc analyses of pooled data from five phase III clinical trials on belimumab in patients with SLE. We summarize the key results below.
The Lupus hub has previously reported on a post-hoc analysis of renal and neuropsychiatric outcomes from these five phase III trials.
Methods
The post-hoc analyses was conducted on the below five randomized, placebo-controlled, phase III trials:
- BLISS-52 (NCT00424476; N = 577)
- BLISS-76 (NCT00410384; N = 548)
- Northeast Asia (NCT01345253; N = 677)
- BLISS-SC (NCT01484496; N = 836)
- EMBRACE (NCT01632241; N = 448)
Included patients were treated with belimumab (10 mg/kg/month intravenously or 200 mg/week subcutaneously) or placebo, alongside standard therapy for 52 weeks. Parodis et al.1 analyzed the attainment of the definition of remission in SLE (DORIS) and Lupus Low Disease Activity State (LLDAS) with belimumab, while Sheikh et al.2 evaluated the efficacy of belimumab by race and ethnicity.
Key findings
Among 1,869 patients treated with belimumab and 1,217 with placebo, 94.4% were female, with a mean age of 37 years. The racial subgroups at baseline comprised 36% Asian, 33% White, 21% Black African ancestry, 10% American Indian or Alaskan Native, <0.1% Native Hawaiian or Other Pacific Islander, while the ethnic distribution comprised 27% Hispanic/Latino and 73% non-Hispanic/Latino.
At Week 52, treatment with belimumab compared with placebo, resulted in:
- Greater attainment of DORIS and LLDAS (Figure 1A), noticeable as early as 20 and 24 weeks, respectively1.
- Early attainment of LLDAS in patients with baseline Systemic Lupus Erythematosus Disease Activity Index 2000 score ≥10 and anti-dsDNA positivity/low complement 3/complement 4 was observed with belimumab compared with the overall population1.
- Higher proportions of patients to be SLE Responder Index-4 (SRI-4) responders in all subgroups by race and ethnicity, except for patients of Black African ancestry (Figure 1A)2.
- Greater differences in time to first severe SFI (Safety of Estrogens in Lupus Erythematosus National Assessment–Systemic Lupus Erythematosus Disease Activity Index Flare Index) flares and any SFI flares in all subgroups except for patients of Black African ancestry (Figure 1B)2.
- Greater differences in time to first British Isles Lupus Assessment Group 1A/2B flares in all subgroups except for patients of White or Black African ancestry (Figure 1B)2.
Figure 1. Attainment of A DORIS, LLDAS, and SRI-4 response and B time to first flares at Week 52*
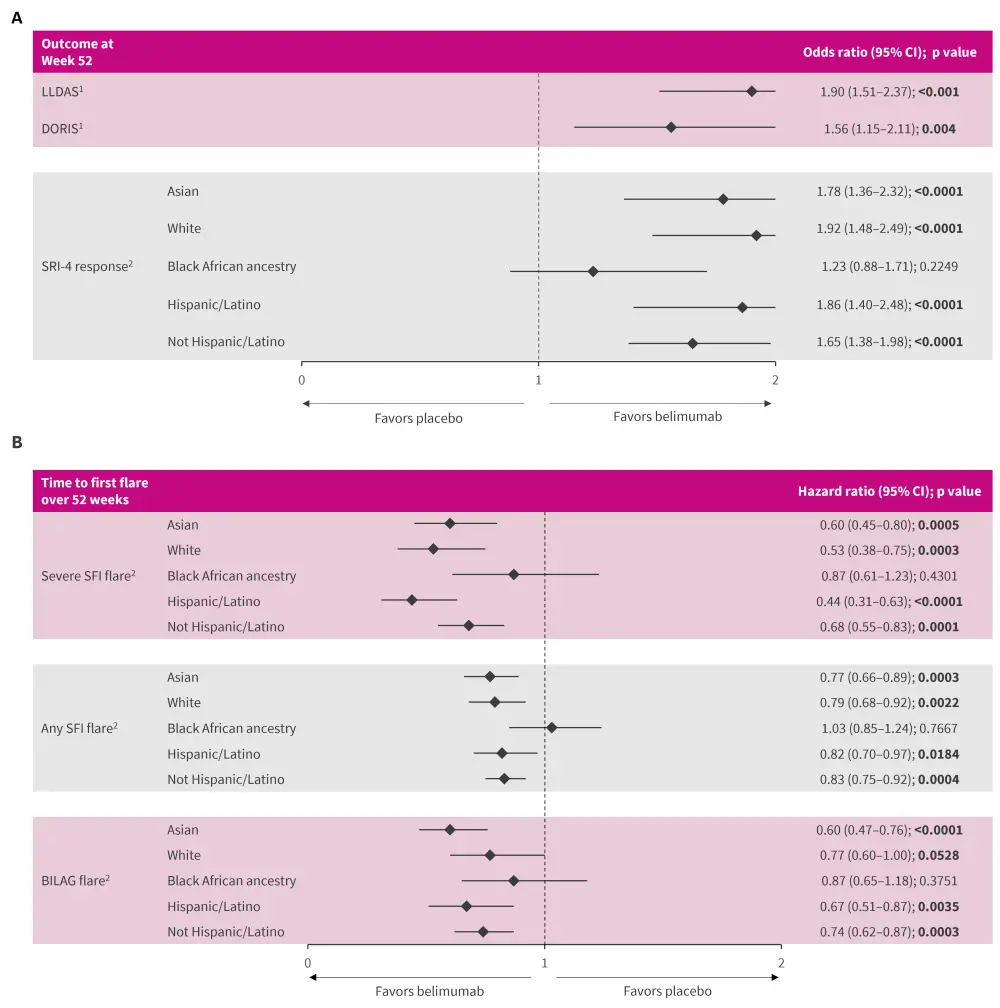
BILAG, British Isles Lupus Assessment Group; CI, confidence interval; DORIS, definition of remission in systemic lupus erythematosus; LLDAS, Lupus Low Disease Activity State; SFI, Safety of Estrogens in Lupus Erythematosus National Assessment (SELENA)–Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) Flare Index; SRI-4, Systemic Lupus Erythematosus Responder Index-4.
*Adapted from Parodis, et al.1 and Sheikh, et al.2
|
Key learnings |
|---|
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


