All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Predictors and prevention of renal flares: Pooled analysis of four phase III trials of belimumab
Renal involvement occurs in 30–60% of the patients with systemic lupus erythematosus (SLE) and remains a substantial contributor to end-stage kidney disease, dialysis, and mortality.1,2 However, there is uncertainty in the identification of circumstances leading to renal flares and belimumab’s efficacy in preventing them.1,2
Below, we summarize a pooled analysis of four phase III trials of belimumab assessing predictors of renal flares in patients with SLE, published by Jägerback et al.1 in Rheumatology; and on their prevention using belimumab alongside antimalarials, published by Gomez et al.2 in Rheumatology.
Methods1,2
- A post-hoc analyses of four phase III trials of belimumab in patients with active extrarenal SLE:
-
- BLISS-52, NCT00424476
- BLISS-76, NCT00410384
- BLISS-Northeast Asia, NCT01345253
- BLISS-SC, NCT01484496
- Patients were administered different doses of belimumab (intravenous [IV] 1 mg/kg, IV 10 mg/kg, and subcutaneous 200 mg) or placebo on top of standard therapy.
- Patients received follow-ups from Weeks 52–76. Predictors of renal flares were assessed using proportional hazards regression analysis,1 and the effect of antimalarials and different doses of belimumab on renal flares was assessed using Cox regression analysis.2
Key findings1,2
- Of 3,225 patients with SLE, 192 developed ≥1 renal flare, with the first occurring after a median follow-up of 197 days.
- The patients had a mean age of 36.7 years, with 94% being women, and 54.6% had current/former renal involvement at baseline.
- The most robust determinants of impending renal flares were current/former renal involvement (hazards ratio [HR]: 15.4; 95% confidence interval [CI], 8.3–28.2; p < 0.001], low serum albumin levels, proteinuria, and low C3 levels at baseline (Figure 1)
- Anti-dsDNA and anti-Smith positivity were also associated with renal flares (Figure 1); however, the association reduced after adjustments
Figure 1. Predictors of renal flares*
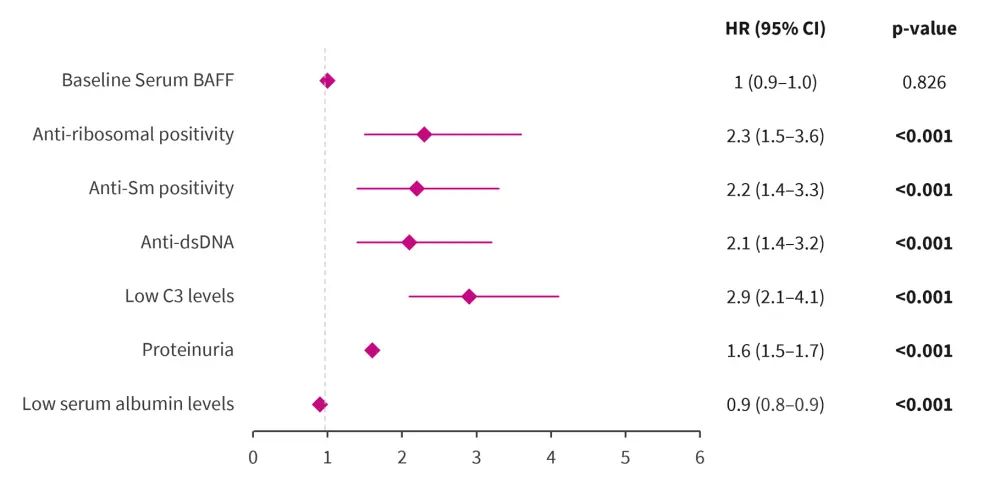
BAFF, B-cell activating factor; CI, confidence interval; dsDNA, double-stranded DNA; HR, hazard ratio.
*Adapted from Jägerback, et al.1
- Treatment with belimumab IV 10 mg/kg and 1 mg/kg resulted in a significantly reduced risk of renal flares (HR, 0.62; 95% CI, 0.41–0.92; p = 0.018 and HR, 0.42; 95% CI, 0.22–0.79; p = 0.007, respectively), while no significant association was found for subcutaneous 200 mg, compared with placebo.
- While antimalarials led to a lower hazard of renal flares (HR, 0.66; 95% CI, 0.55–0.78; p < 0.001), the prevention was conferred when antimalarials were coadministered with belimumab, particularly with the IV 1 mg/kg dose (Figure 2).
Figure 2. Incidence rate of renal flares comparing different belimumab doses with and without antimalarials*
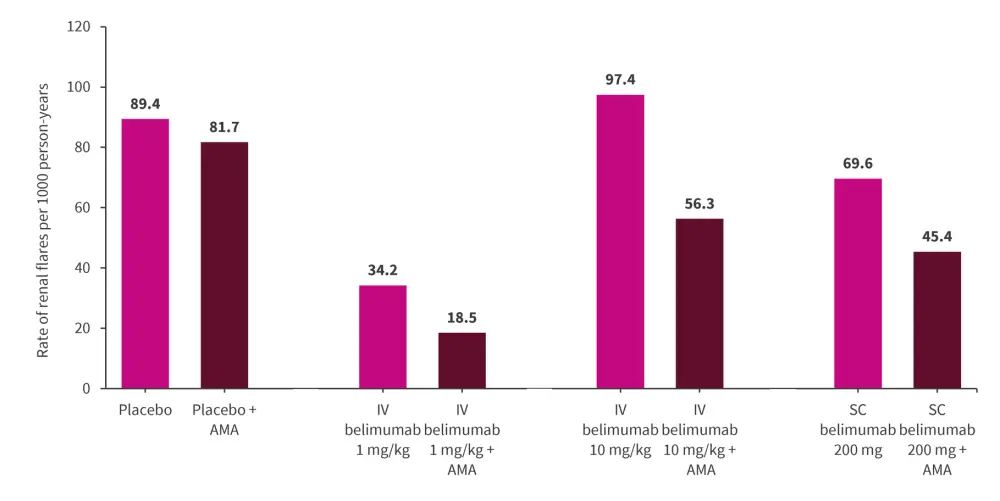
AMA, antimalarial; IV, intravenous; SC, subcutaneous.
*Data from Gomez, et al.2
|
Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



