All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Case series: Telitacicept in patients with SLE and LN after suboptimal response to belimumab
Belimumab inhibits B-cell activating factor and is approved for the treatment of patients with systemic lupus erythematosus (SLE) and lupus nephritis, alongside standard therapy, by the U.S. Food and Drug Administration (FDA).1,2 However, the treatment can be ineffective for some patients.3 Telitacicept inhibits both B-cell activating factor and a proliferation-inducing ligand simultaneously and is approved in China for the treatment of active SLE.1,3
Below, we summarize the efficacy and safety of telitacicept in cases published by Fan et al. in Zeitschrift für Rheumatologie1 and Huang et al. in Lupus,3 where belimumab did not yield an adequate response.
Methods1
For the case series by Fan et al., the records of patients diagnosed with SLE at the Wuhan Hospital of Chinese and Western Medicine, China, between March 2022 and July 2023, were assessed.
Key findings1,3
Case series by Fan et al.1
A total of 14 patients with refractory SLE were treated with telitacicept 160 mg (n = 7) and 80 mg (n = 7) after suboptimal response with belimumab plus standard therapy. The mean age was 32.9 years; 79% were female. Before telitacicept, five patients received ≥2 immunosuppressants, and all had discontinued belimumab for ≥6 months due to persistent and recurrent conditions and difficulty in tapering steroids.
Treatment with telitacicept for an average of 34.1 (17–62) weeks (observational endpoint) resulted in:
- total SLE Responder Index-4 response rate of 78.9%
- reduction of the mean SLE Disease Activity Index score from 8.6 at baseline to 4.3 (Figure 1A)
- 100% achievement of no additional organ reaching British Isles Lupus Assessment Group Grade A or ≤1 additional organ elevating to Grade B
- no increase of Physician Global Assessment ≥0.3, suggesting stability or improvement without deterioration (Figure 1B)
- tapering of glucocorticoid use by >25% or a maintenance dose of ≤7.5 mg/day in 12 cases and no increase in glucocorticoid dosage in any of the patients (Figure 1C)
- normalized complement levels in seven cases
- reduction in 24-hour urinary protein value in 13 cases
- normalized plasma albumin levels in four cases
- negative anti-dsDNA antibody titer in three out of seven cases subjected to reexamination
- non-significant reduction of serum total immunoglobulin (Ig) in nine patients, serum IgG in four patients, and peripheral blood lymphocyte in six patients
- no serious infection
- restoration of normal liver function in a refractory patient with lupus hepatitis where belimumab plus standard treatment for 6 months proved ineffective
Figure 1. Reduction in A SLEDAI score, B PGA score, and C glucocorticoid use in patients receiving telitacicept after inadequate response with belimumab*
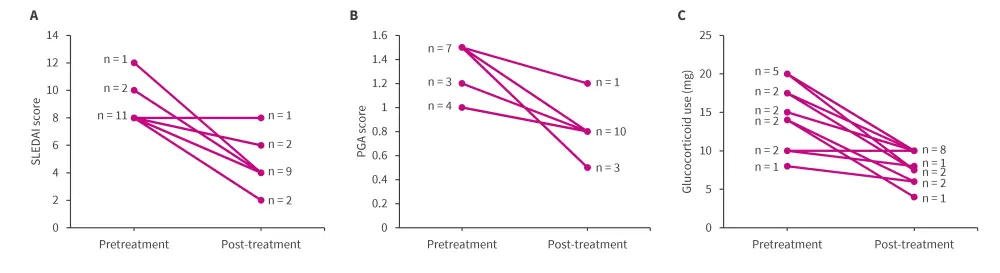
PGA, Physician Global Assessment; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index.
*Data from Fan, et al.1
A case report by Huang et al.3
Figure 2 elucidates a case report, wherein SLE, unresponsive to ten doses of belimumab, was effectively controlled following a switch to telitacicept.
Figure 2. Clinical case overview of a 35-year-old female*
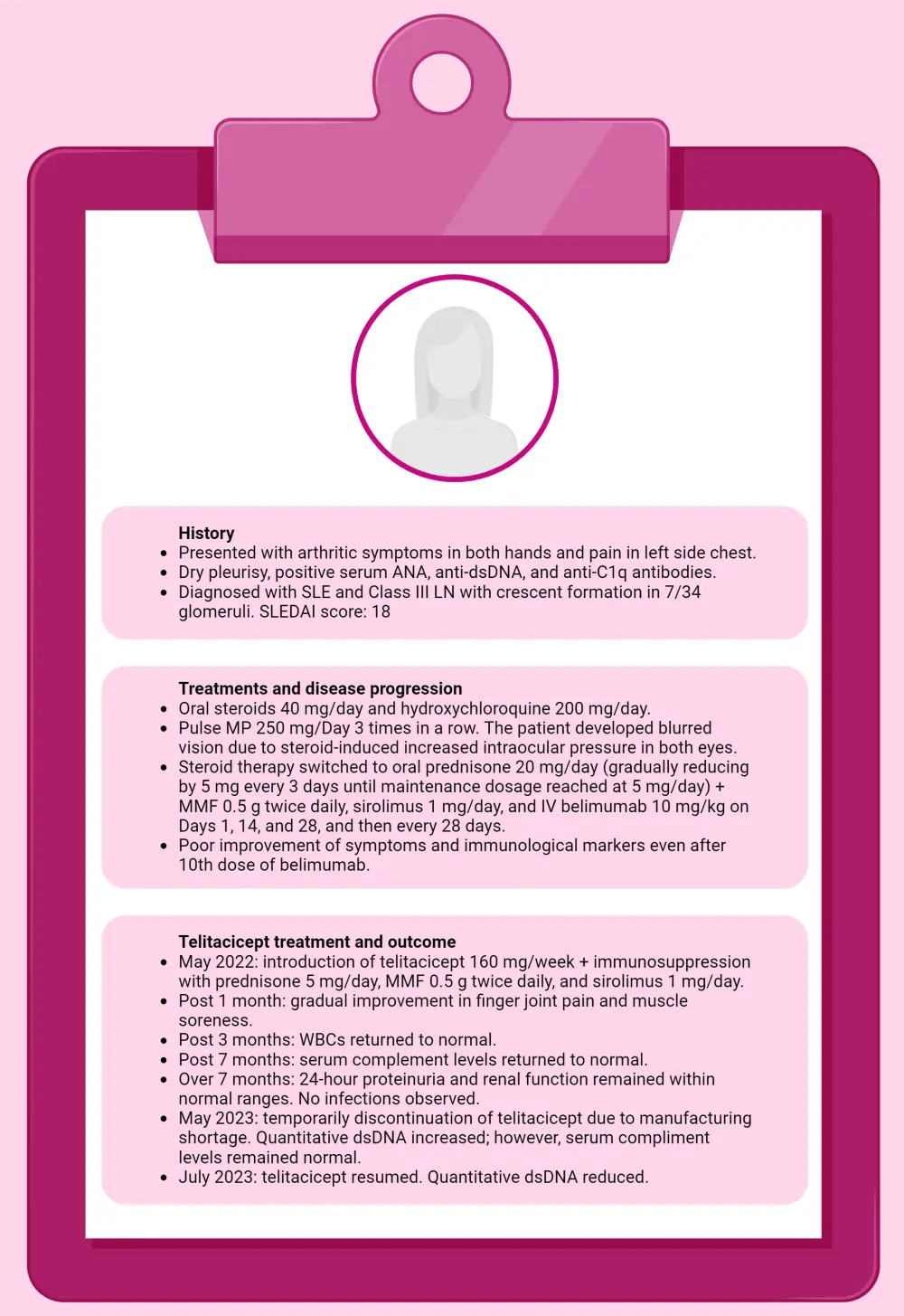
ANA, anti-nuclear antibody; CT, computed tomography; dsDNA, double-stranded DNA; MMF, mycophenolate mofetil; MP, methylprednisolone; WBC, white blood cell.
*Data from Huang, et al.2
| Key learnings1,3 |
|
Telitacicept appears to be an effective and safe alternative treatment in patients with SLE who show inadequate response to belimumab. |
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



