All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Efficacy and safety of baricitinib in patients with SLE: Pooled meta-analysis of RCTs
Baricitinib, a selective Janus kinase inhibitor (JAKi), is approved for the treatment of certain autoimmune and inflammatory conditions. However, its efficacy and safety in patients with systemic lupus erythematosus (SLE) remains uncertain.1
The Lupus Hub previously presented the efficacy and safety of baricitinib from the phase III SLE-BRAVE trials. Below, we summarize a systematic literature review and meta-analysis of randomized clinical trials (RCTs) investigating the efficacy and safety of baricitinib 4 mg and 2 mg in patients with SLE, published by Amer et al. in Clinical Rheumatology.1
Methods
A systematic literature search was performed on Scopus, PubMed, Cochrane Central Register of Controlled Trials, Web of Science, Google Scholar, and ClinicalTrials.gov from inception until April 27, 2023. The outcomes were pooled as the risk ratio using a random effects model.
The primary outcome was SLE Responder Index-4 (SRI-4) response.
Results
The study included three RCTs, comprising 1,849 patients with SLE. The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) flow diagram and characteristics of the selected studies are shown in Figure 1.
Figure 1. A PRISMA flow diagram and B summary of the selected studies*
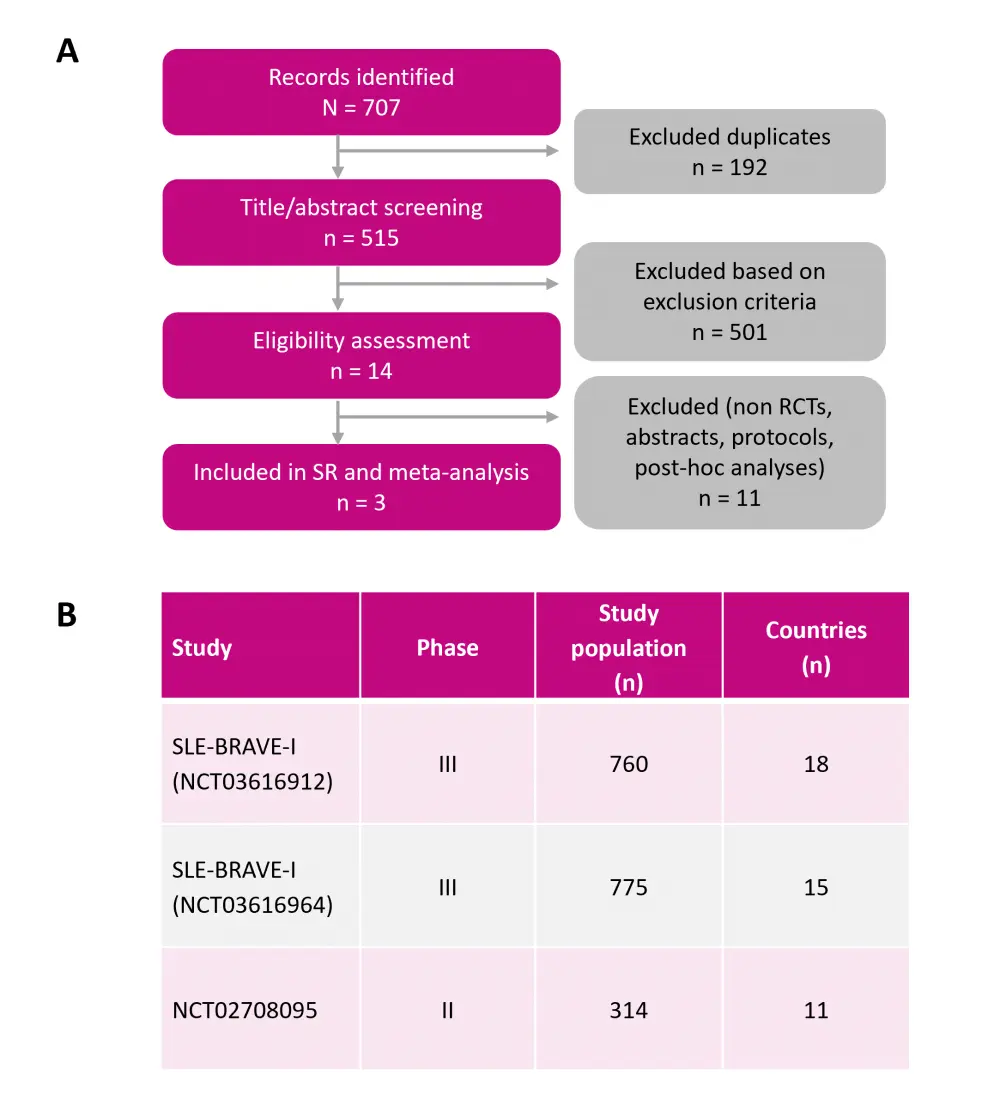
PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analysis; SLE, systemic lupus erythematosus; SR, systematic review.
*Adapted from Amer, et al.1
Efficacy
Compared with placebo, treatment with baricitinib 4 mg led to a significantly higher proportion of patients with SLE achieving an SRI-4 response at Week 24, but not at Week 52. However, baricitinib 2 mg did not show significant differences in SRI-4 response at both Week 24 and Week 52 (Figure 2A).
Baricitinib 4 mg was also associated with a significant reduction in the SLE Activity Index 2000 score (≥4 points from baseline) and SLE Activity Index 2000 remission of arthritis or rash. The remaining secondary outcomes did not reach statistical significance, regardless of baricitinib dosage.
Safety
Baricitinib 4 mg was associated with significantly a higher risk of serious infection and serious adverse events compared with placebo (Figure 2B). Other safety findings were not statistically significant at either dose compared with placebo.
Figure 2. Pooled results from RCTs on A efficacy and B safety of baricitinib*
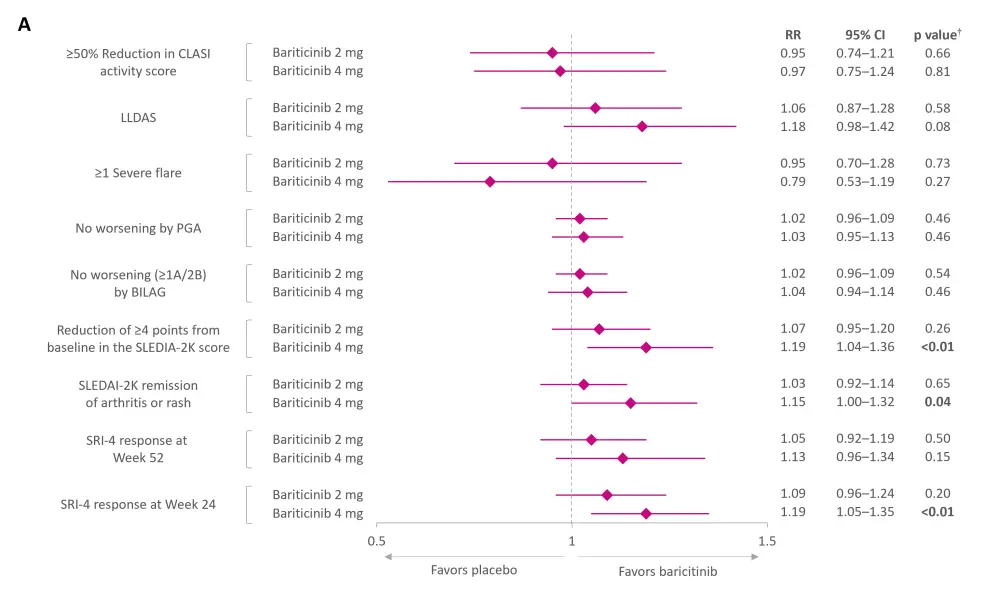
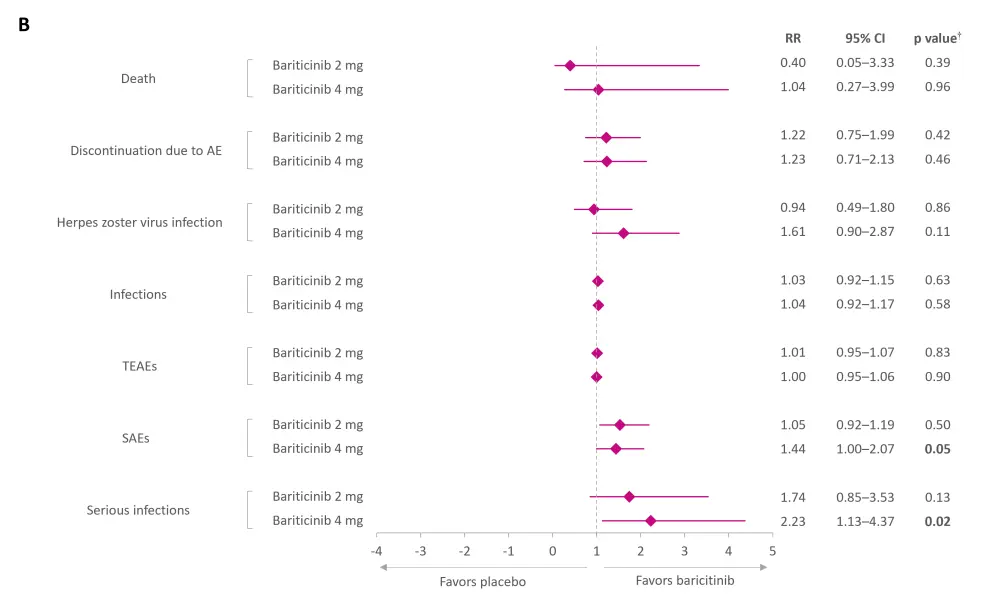
AE, adverse event; BILAG, British Isles Lupus Assessment Group; CI, confidence interval; CLASI, Cutaneous Lupus Erythematosus Disease Area and Severity Index; LLDAS, Lupus Low Disease Activity Score; PGA, Physician’s Global Assessment of Disease Activity; RR, risk ratio; SAE, serious AE; SLE, systemic lupus erythematosus; SRI-4, SLE Responder Index-4; SLEDAI-2K, SLE Activity Index 2000; TEAE, treatment-emergent AE.
*Data from Amer BE, et al.1
†Values in bold are statistically significant.
Conclusion
Despite the termination of the SLE-BRAVE-X phase III trial and unfavorable outcomes from the SLE-BRAVE-II trial, this meta-analysis revealed that baricitinib 4 mg could potentially reduce SLE disease activity, especially in patients presenting articular symptoms. In contrast, baricitinib 2 mg did not show any clinical benefit and was consistent with previous findings.
Further research is required to evaluate the long-term efficacy of baricitinib, its effectiveness in patients with cutaneous lupus erythematosus, and variations in effects over time. While waiting for further robust evidence, it is advisable to assess the benefits and risks involved with treatment with baricitinib, particularly in patients with SLE who are at higher risk of recurrent or chronic infections.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

