All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Novel urinary biomarkers for determining active renal involvement in systemic lupus erythematosus
Lupus nephritis (LN) is the most prevalent serious complication of systemic lupus erythematosus (SLE). Given the limitations with existing diagnostic procedures, there’s an urgent need for a reliable biomarker with high predictive value for LN. Li et al.1 recently published an article in Journal of Autoimmunity, identifying and validating novel urinary biomarkers capable of distinguishing active renal involvement in SLE. Below, we summarize their key findings.
Methods
- A proximity extension assay (PEA)-based approach was used to screen archived urine samples from 117 subjects (age range: 18–74 years), including:
- 34 patients with active renal (AR) lupus;
- 27 patients with active non-renal lupus;
- 26 patients with inactive SLE (iSLE); and
- 30 healthy controls
- Validation was done using enzyme-linked immunosorbent assay (ELISA) in an independent, ethnically diverse cohort.
- Proteomic data was cross-referenced to renal single-cell transcriptomic data to elucidate potential tissue and cellular origins of biomarkers.
- Receiver operating characteristic (ROC) curve was employed to evaluate the performance of biomarkers in discriminating between groups.
Key findings
Proteomic screening of LN urine
- Of the 543 distinct human proteins screened using PEA, 327 urinary proteins were found to be significantly elevated in AR vs iSLE (p < 0.05).
Functional pathway enrichment in 327 significantly changed proteins:
- Compared with iSLE, the AR subjects demonstrated key enrichments in:
- Gene ontology (GO) biological processes: lymphocyte and monocyte chemotaxis, along with chemokine-mediated signaling pathways;
- Cellular components: external side of plasma membrane, collagen-containing extracellular matrix, and endoplasmic reticulum lumen;
- GO molecular functions: CCR chemokine receptor binding, chemokine activity, virus receptor activity, and other functions; and
- Kyoto Encyclopedia of Genes and Genomes pathways: cytokine-cytokine receptor interactions.
Validation of urine proteins using ELISA:
- Eight proteins exhibited substantial fold-change elevations in distinguishing AR from iSLE (Figure 1).
- The six most discriminatory urine biomarkers for AR lupus were ICAM-2, FABP4, FASLG, IGFBP-2, SELE, and TNFSF13B/BAFF, with ROC area under the curve (AUC) values ≥80%, outperforming the conventional biomarkers (C3, C4, and anti-DNA), which have ROC AUC values averaging 0.55.
- Furthermore, these proteins exhibited good correlation with SLE Disease Activity Index (SLEDAI), renal SLEDAI (rSLEDAI), and proteinuria, with the most significant correlations observed in urine IL-1RT2, ICAM-2, IGFBP-2, FABP4, and SELE.
Figure 1. Validation of novel urine biomarkers by ELISA*
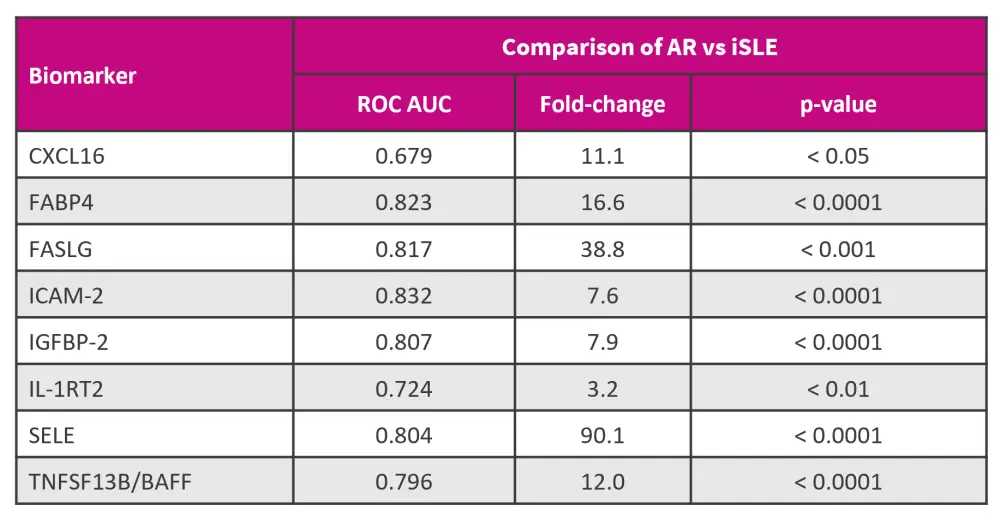
AR, active renal; AUC, area under the curve; iSLE, inactive systemic lupus erythematosus; ROC, receiver operating characteristic.
*Adapted from Li, et al.1
Potential tissue origin of elevated urinary biomarkers
- Gene expression profiles in lupus peripheral blood mononuclear cells revealed the following:
- CXCL16, IL-1RT2, and TNFSF13B/BAFF were highly expressed in monocytes and dendritic cells.
- ICAM-2 was ubiquitously expressed in various cell types but was highly expressed in monocytes, dendritic cells, and platelets.
- FASLG was highly expressed in natural killer/T cells.
- RNA level analysis within LN kidneys suggested elevated levels of ICAM-2 in both glomerular and tubulo-interstitial compartments. CXCL16 and FABP4 showed elevated expression in LN glomeruli in most datasets, with similar trends in the tubulo-interstitial regions.
|
Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



