All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Editorial theme | Patient-reported outcome measures in SLE
Do you know... Which of the following generic patient-reported outcome tools can be used to assess Type 2 SLE symptoms, such as fatigue, myalgia, mood disturbances, and cognitive impairment, and assess a patient’s flare status?
Patients living with systemic lupus erythematosus (SLE) experience significantly declined health-related quality of life (HRQoL), with fatigue, pain, and depression being among the common manifestations of the condition.1 In a survey of 500 patients with SLE in the United States, the most frequently reported symptoms were fatigue (69%), joint stiffness (57%), sleep problems (55%), pain or swelling in joints (53%), and muscle pain (52%); the majority of symptoms were reported to be of moderate to severe intensity.2
Recently, the concept of Type 1 and Type 2 SLE symptoms has been introduced. The Type 1 symptoms are considered inflammatory, pertaining to autoimmune dysfunction and organ damage; whereas Type 2 is characterized by symptoms typically observed in fibromyalgia, such as fatigue, myalgia, mood disturbances, and cognitive impairment. Type 2 SLE symptoms are generally less responsive to traditional immunosuppression; therefore, it is crucial to identify and acknowledge them to guide appropriate management.1
Disease activity and damage are insufficient outcome measures for some of the most concerning symptoms for patients with SLE. Incorporating patient-reported outcome (PRO) tools in SLE care can facilitate the acknowledgment, quantification, and documentation of symptoms, potentially bridging the gap between physician assessment and patient satisfaction.1,2 Various organizations, such as the European Alliance of Associations for Rheumatology, the Outcome Measures in Rheumatology Clinical Trials, American College of Rheumatology, and the World Health Organization now advocate for the assessment of HRQoL and the incorporation of PRO measures in clinical trials and routine care.1
In our editorial theme series on treatment goals and guidelines in patients with lupus, we are pleased to introduce our fourth article focusing on PRO tools, both generic and disease specific, highlighting their significance in both clinical trials and routine care. The first two articles in this series discussed treat-to-target in SLE and outcome measures in SLE: the current landscape and future perspectives. We have also reported an expert discussion covering LLDAS versus remission as treat-to-target goals.
SLE-specific PRO tools
Currently, the available PRO tools for measuring HRQoL in patients with SLE include:1
- Lupus Quality of Life (LupusQoL);
- Lupus Patient-Reported Outcome (LupusPRO);
- SLE-Specific Quality of Life (SLEQoL);
- Lupus Quality of Life (L-QoL); and
- Lupus Impact Tracker (LIT).
Each of these tools has been well-validated for their application in patients with SLE. Figure 1. provides a comprehensive summary on the characteristics, number of items/questionnaires, domains, response technique, strengths, limitations, and utility of these tools in both research and routine care settings.
Figure 1. SLE-specific PRO tools*
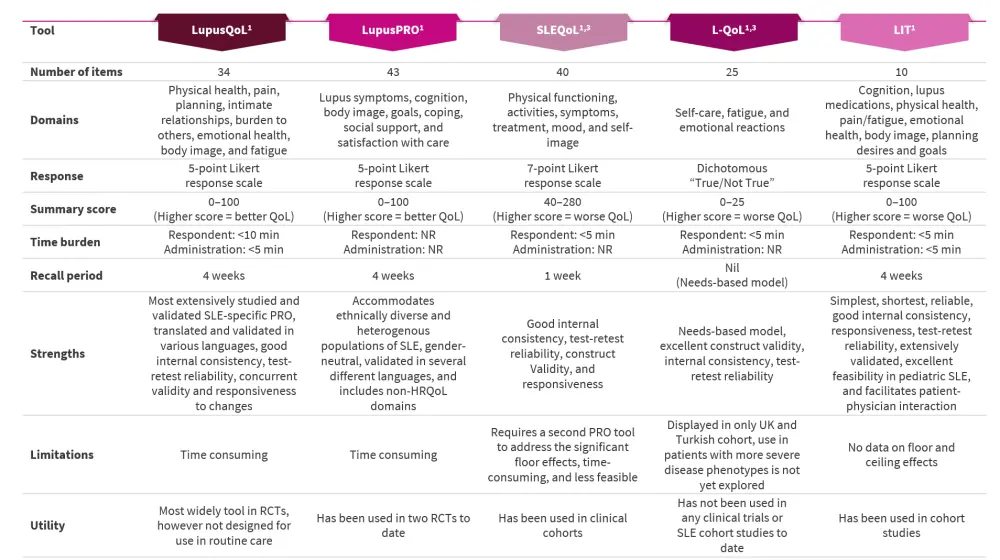
HRQoL, health-related quality of life; LIT; Lupus Impact Tracker; L-QoL, Lupus Quality of Life Questionnaire; LupusPRO; Lupus Patient-Reported Outcome; LupusQOL, Lupus Quality of life; NR, not reported; PRO; patient-reported outcome; RCT, randomized clinical trial; QoL, quality of life; SLE; systemic lupus erythematosus, SLEQoL, SLE quality of life; UK, United Kingdom.
*Adapted from Nguyen, et al.1
Generic PRO tools widely utilized in SLE
While generic PRO tools were not specifically designed to measure HRQoL in a particular disease condition, some of these tools have been widely utilized in SLE management as their domains align with those relevant to lupus-related QoL. Additionally, they have the advantage of enabling comparison with other disease states, such as rheumatoid arthritis and fibromyalgia.1
The most common generic PRO tools that are widely used in SLE research and clinical care are:1
- 36-item Short-Form Health Survey (SF-36);
- EuroQoL-5D (EQ-5D);
- Multi-Dimensional Health Assessment Questionnaire (MDHAQ); and
- Patient-Reported Outcomes Measurement Information System (PROMIS).
Functional Assessment of Chronic Illness Therapy-Fatigue, Work Productivity and Activity Impairment, Worst Pain Numerical Rating scale, and Worst Joint Pain Numerical Rating scale are few other tools utilized to measure fatigue, pain, and work productivity and activity impairment in patients with SLE.2 Figure 2 presents a comprehensive overview of the generic PRO tools utilized in SLE.
Figure 2. Generic PRO tools utilized in SLE management*
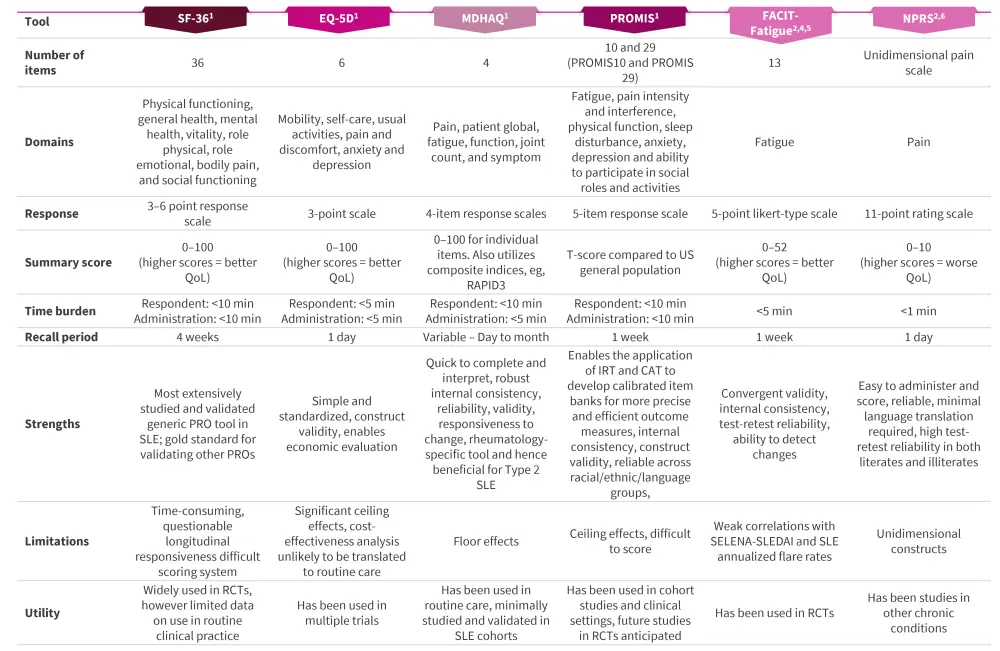
CAT, computerized adaptive testing; EQ-5D, EuroQoL-5D; FACIT, Functional Assessment of Chronic Illness Therapy; IRT, item response theory; MDHAQ, Multi-Dimensional Health Assessment Questionnaire; NPRS, Numerical Pain Rating Scale; PRO, patient-reported outcome; PROMIS, Patient-Reported Outcomes Measurement Information System; RCT, randomized clinical trial; SELENA-SLEDAI, Safety of Estrogens in Lupus Erythematosus National Assessment-Systemic Lupus Erythematosus Disease Activity Index; SF-36, 36-item Short-Form Health Survey; SLE, systemic lupus erythematosus; QoL, quality of life.
*Adapted from Nguyen, et al.1
Conclusion
There are various well-validated generic and SLE-specific PRO tools that have proven utility in clinical trials and complement disease activity and damage measures in routine care.1
However, there is no universally accepted standard measure capable of capturing all aspects of HRQoL in patients with SLE. There is also a need for improved awareness and understanding of the United States Food and Drug Administration (FDA) PRO guidance recommendations, ensuring these measures adhere to the guidance requirements.7
Despite the existing limitations, integrating PROs into disease management is crucial to allow physicians to effectively quantify, comprehend, and address patient perspectives and experiences with their respective conditions and treatment, and thus overcome physician-patient discordance.1
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



