All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Management of lupus nephritis: KDIGO 2024 Clinical Practice Guidelines
Since the Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Glomerular Diseases was updated in 2021,1 there have been advances in the treatment of lupus nephritis (LN), including the approval of belimumab and voclosporin by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) as add-on immunosuppressives to standard-of-care LN treatment.2
Recently, KDIGO has issued an evidence-based update on recommendations for managing LN, summarized by Rovin et al.2 in Kidney International. Here, we provide a summary of the guidelines.
Methods3
An evidence review team updated the systematic evidence review, using their expertise in guideline development methodology, and a work group was established by KDIGO for writing recommendations and practice points. Then, following a public review and incorporating external feedback, the guidelines were finalized and published.
Key findings2,3
Recommendations
Recommendation statements were formulated for the general management of LN and the initial and maintenance therapy for active Class III/IV LN (Figure 1A). The practice points for all the classes of LN are outlined in Figure 1B.
Figure 1. A Recommendations and B practice points for management of patients with LN*
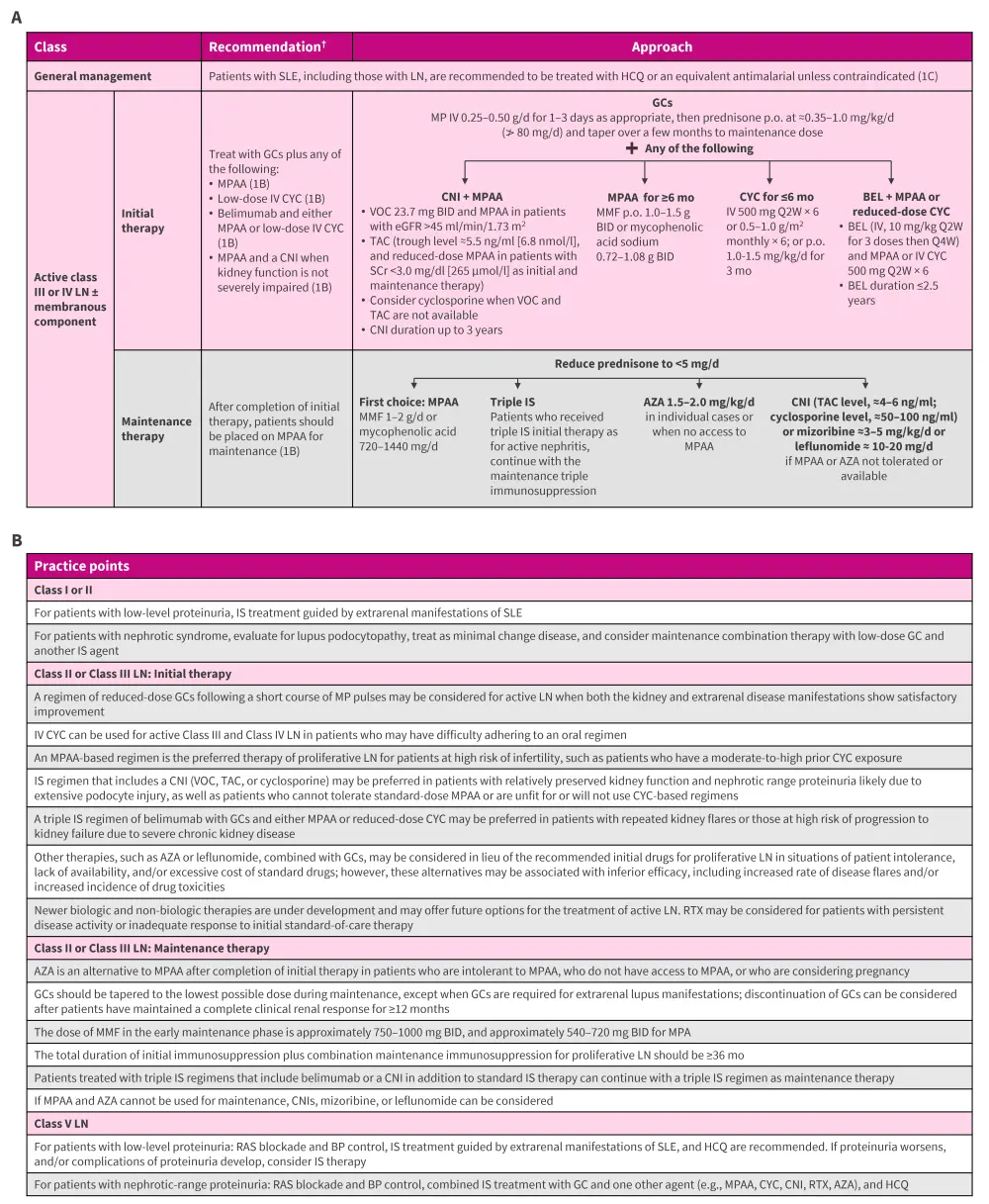
AZA, azathioprine; BEL, belimumab; BID, twice-daily; BP, blood pressure; CNI; calcineurin inhibitor; CYC, cyclophosphamide; d, day; eGFR, estimated glomerular filtration rate; GC, glucocorticoid; HCQ, hydroxychloroquine; IV, intravenous; IS, immunosuppressive; KDIGO, Kidney Disease: Improving Global Outcomes; LN, lupus nephritis; MMF, mycophenolate mofetil; MP, methylprednisolone; MPAA, mycophenolic acid analog; mo, month; p.o., oral administration; Q2W, every 2 weeks; Q4W, every 4 weeks; RAS, renin-angiotensin system; RTX, rituximab; SCr, serum creatinine; SLE, systemic lupus erythematosus; TAC, tacrolimus; VOC, voclosporin.
*Adapted from Rovin, et al.2; KDIGO Lupus Nephritis Work Group.3
†The certainty of evidence was graded on a scale of A to D, indicating high to very-low certainty, respectively, and recommendations were graded as Level 1 (“we recommend”) or Level 2 (“we suggest”).
Practice points for management of LN in special situations
Lupus nephritis and thrombotic microangiopathy
- Systemic lupus erythematosus-associated thrombotic thrombocytopenic purpura: plasma exchange + glucocorticoids + rituximab ± caplacizumab.
- Primary or secondary complement-mediated thrombotic microangiopathy: consider eculizumab.
- Antiphospholipid syndrome nephropathy: anticoagulation ± plasma exchange.
Pregnancy in patients with LN
- Patients with active LN should be advised to avoid pregnancy while experiencing active disease or when treatment with potentially teratogenic drugs is ongoing, and for ≥6 months after LN becomes inactive.
- To reduce the risk of pregnancy complications, hydroxychloroquine should be continued during pregnancy, and low-dose aspirin should be started before 16 weeks of gestation.
- Safe immunosuppressive treatments during pregnancy include glucocorticoids, hydroxychloroquine, azathioprine, tacrolimus, and cyclosporine.
Treatment of LN in children
Treat pediatric patients with LN using immunosuppression regimens similar to those used in adults, while accounting for factors such as dose adjustment, growth, fertility, and psychosocial factors when planning therapy.
Management of LN patients with kidney failure
- Patients with LN who develop kidney failure may be treated with hemodialysis, peritoneal dialysis, or kidney transplantation.
- Kidney transplantation is preferred to long-term dialysis.
|
Key learnings |
|
The key update in the 2024 KDIGO guidelines is the recommendations for the initial treatment of patients with active Class III/IV ± V LN, which involves using glucocorticoids alongside low-dose intravenous cyclophosphamide or mycophenolic acid analogs (dual immunosuppressive therapy). Alternatively, belimumab or voclosporin (or tacrolimus) can be added (triple immunosuppressive therapy). |
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



