All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Systematic review and meta-analysis on efficacy and safety of JAK inhibitors in patients with S/CLE
Although various Janus kinase (JAK) inhibitors for the treatment of systemic lupus erythematosus (SLE) and cutaneous lupus erythematosus (CLE) have been investigated in randomized clinical trials (RCTs), none have yet been approved for the treatment of lupus. However, emerging case studies and case series demonstrating the efficacy of JAK inhibitors in patients with SLE and CLE emphasize the urgent need for scientific evidence supporting this treatment approach.1
Recently, Ma et al.1 published a systematic review and meta-analysis in Autoimmunity reviews evaluating the efficacy and safety of JAK inhibitors in patients with SLE and CLE. Here, we summarize the key findings.1
Methods1
A search was conducted in PubMed, Embase, Web of Science, and the Cochrane Library to identify studies evaluating JAK inhibitors in patients with SLE and CLE until February 28, 2023. Meta-analysis was performed when there were at least three studies with low or moderate risk of bias and similar outcome measures.
Results
The search and study selection flow diagram and key characteristics of the selected studies are shown in Figure 1.
The SLE studies included a total of 2,318 adult patients with a mean age of 42 years. Of these, most patients were female (93.7%). In CLE studies, 142 patients were adults (except for one 3-year-old child with chilblain lupus erythematosus [ChLE]) and the mean age was 46.7 years; 81% of the patients were female.
Figure 1. A PRISMA flow diagram and B summary of the selected studies*
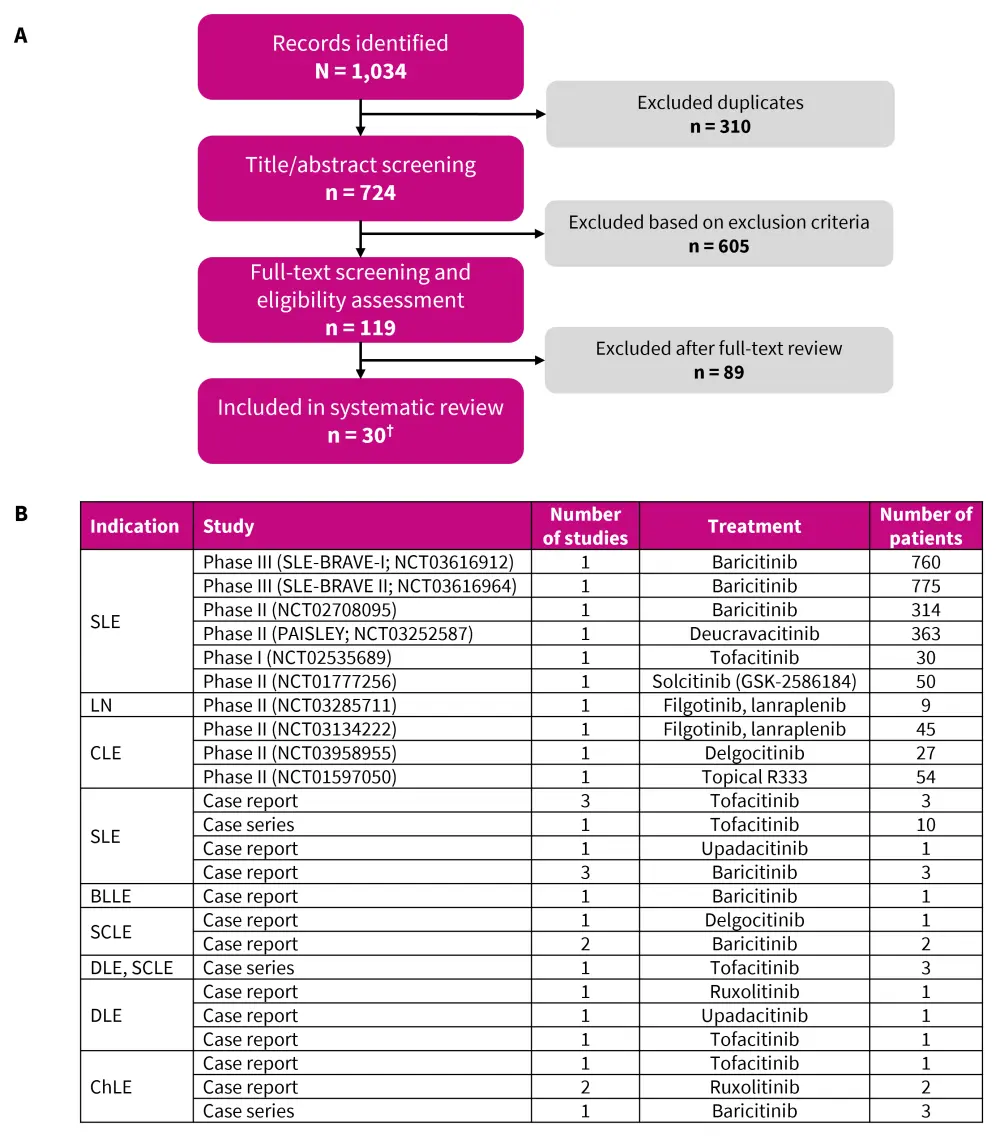
BLLE, blaschko linear lupus erythematosus; ChLE, chilblain lupus erythematosus; CLE, cutaneous lupus erythematosus; DLE, discoid lupus erythematosus; LN, lupus nephritis; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized clinical trial; SCLE, subacute cutaneous lupus erythematosus; SLE, systemic lupus erythematosus.
*Data from Ma, et al.1
†10 RCT’s and 20 case reports and series.
Efficacy
Effect of disease activity
Meta-analysis of seven RCTs showed that JAK inhibitors were more effective in achieving SLE Responder Index-4 response, Lupus Low Disease Activity State, and British Isles Lupus Assessment Group-based Composite Lupus Assessment response in patients with SLE vs placebo (Figure 2). The Lupus Hub has previously reported results from the phase III SLE-BRAVE trials and a pooled meta-analysis of RCTs evaluating the efficacy and safety of baricitinib.
Figure 2. Meta-analysis of JAK inhibitors versus placebo in patients with SLE*
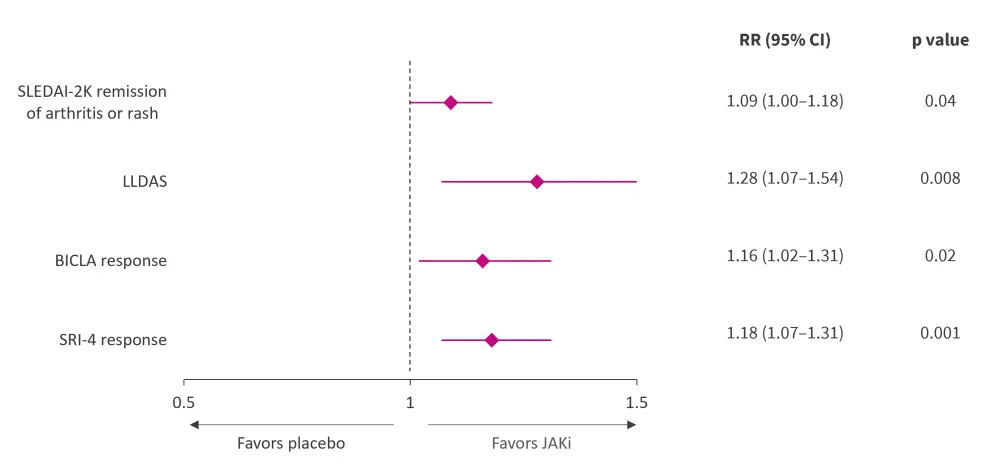
BICLA, British Isles Lupus Assessment Group-based Composite Lupus Assessment; CI, confidence interval; JAK, Janus kinase; LLDAS, Lupus Low Disease Activity State; RR, risk ratio; SAE, serious adverse event; SLE, systemic lupus erythematosus; SLEDAI-2K, SLE Disease Activity Index 2000; SRI, SLE Responder Index.
*Adapted from Ma, et al.1
Effect on organ involvement in SLE
Meta-analysis of five RCTs showed that baricitinib was more effective than placebo for SLE Disease Activity Index 2000 (SLEDAI-2K) remission of arthritis or rash, particularly in musculoskeletal and mucocutaneous systems (Figure 2).
A phase II trial (NCT03252587) demonstrated that deucravacitinib showed significant improvement in organ-specific endpoints compared with placebo. Tofacitinib demonstrated alleviation of arthritis symptoms in a case series of four patients. Two patients experiencing Jaccoud’s arthropathy reported improvement in joint symptoms after treatment with upadacitinib and tofacitinib.
Filgotinib showed a median reduction of 50.7% in 24-hour urine protein and 51.6% in spot ‘urine protein creatinine ratio’ after 16 weeks vs lanraplenib, including complete remission in two out of four patients.
Effect on CLE
Primary and secondary outcomes were not met in two-phase II trials assessing the efficacy of topical delgocitinib and R333 in the treatment of discoid lupus erythematosus (DLE). In addition, the primary outcome of change from baseline in CLE Disease Area and Severity Index-activity score at Week 12 was not met in a trial evaluating filgotinib in moderate-to-severe CLE.
In clinical settings, 16 patients with CLE experienced positive outcomes with JAK inhibitors. Of the two patients experiencing alopecia, the patient treated with topical 1.5% ruxolitinib showed slight hair regrowth, while the other reported halted progression of frontal fibrosing alopecia after treatment with baricitinib 4 mg/day. Four of five patients with DLE reported improvement in skin lesions with topical and oral JAK inhibitors. Out of the four patients with subacute CLE, three achieved complete remission of rash.
Six patients with ChLE showed rapid clinical improvement within 1–8 months with baricitinib, ruxolitinib, and tofacitinib, while one patient had a complete remission of all lesions when treated with ruxolitinib 40 mg daily.
Safety
All the studies reported a low incidence of adverse events (AEs), mostly involving mild infections in the upper respiratory tract and urinary system. Overall, JAK inhibitors had a similar incidence of AEs and serious AEs compared with placebo (Figure 3).
Figure 3. Meta-analysis of JAK inhibitor safety versus placebo in patients with SLE*
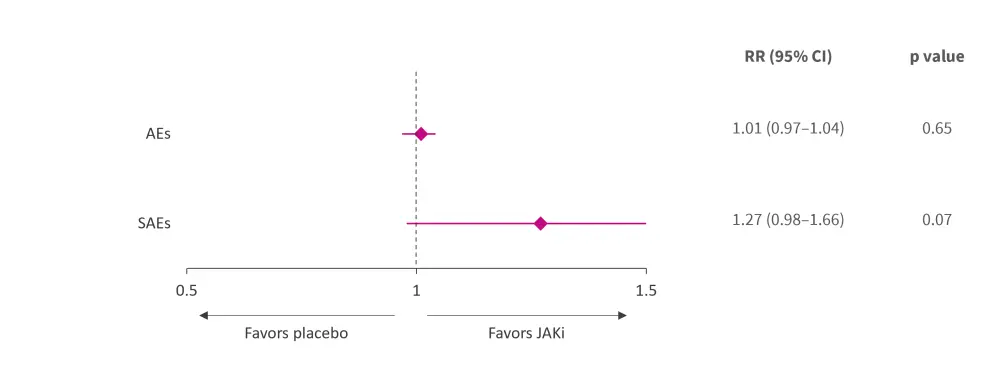
AE, adverse event; CI, confidence interval; JAKi, Janus kinase inhibitor; RR, risk ratio; SAE, serious adverse event; SLE, systemic lupus erythematosus.
*Adapted from Ma, et al.1
Conclusion
The results from this systematic review and meta-analysis indicate that JAK inhibitors have a promising efficacy in SLE and CLE, particularly in treating cutaneous and musculoskeletal manifestations; the safety profile was also acceptable. However, these findings should be interpreted in the context of certain limitations of the study. The included studies showed variability in efficacy, and some studies showed varying degree of bias. Lack of quantitative data in case reports and series, and the non-reporting of failed studies, made assessment of JAK inhibitor efficacy even more challenging. Future studies are warranted to evaluate the efficacy of JAK inhibitors on CLE, lupus nephritis, and serological activity.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



