All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The lupus Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lupus Hub cannot guarantee the accuracy of translated content. The lupus and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lupus Hub is an independent medical education platform, supported through a founding grant from AstraZeneca. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lupus content recommended for you
Systemic lupus erythematosus: an overview
Do you know... What is the sex distribution among adults diagnosed with systemic lupus erythematosus?
Systemic lupus erythematosus (SLE) is a multisystem chronic autoimmune disorder with various genetic, epigenetic, socioeconomic, and environmental factors influencing etiology, disease progression, and outcomes. Varying clinical manifestations and prognoses, arising due to the complex immune dysregulation implicated in disease pathogenesis, create challenges for SLE diagnosis and management.1 Here, we provide a summary of the etiology, epidemiology, pathophysiology, clinical manifestations, diagnostic tests and tools, and standard and emerging treatment options available for SLE.
Etiology
The development of SLE involves an intricate interplay between environmental and genetic factors, resulting in epigenetic modifications and contributing to disease onset and progression (Figure 1).2
Figure 1. Risk/trigger factors for SLE*
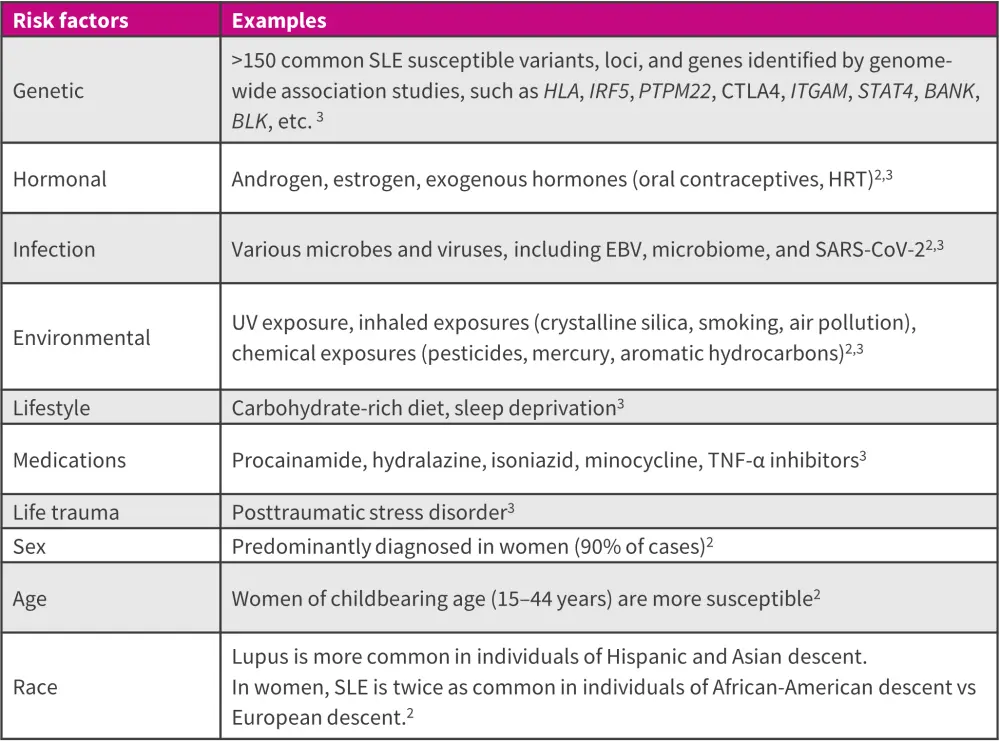
EBV, Epstein-Barr virus; HRT, hormone replacement therapy; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SLE, systemic lupus erythematosus; TNF, tumor necrosis factor; UV, ultraviolet.
*Adapted from Crow, et al.3; and Ameer, et al.2
Epidemiology
Around 3.41 million people worldwide and 1.5 million people in the US are living with lupus. The epidemiology of SLE varies by age and gender, with a higher prevalence among women of childbearing age (Figure 2).4-6
Figure 2. Epidemiology of SLE*
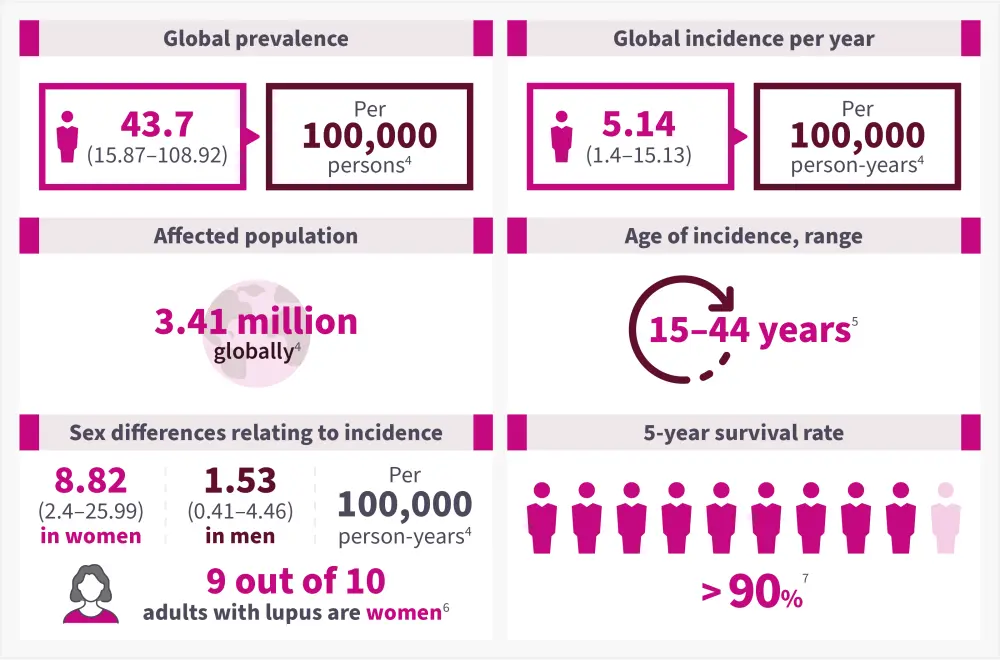
*Data from Tian, et al.4; CDC.5; Lupus Foundation of America.6; and Tsang-A-Sjoe, et al.7
Pathophysiology
The underlying pathophysiology of SLE is poorly understood; however, it is known to involve both innate and adaptive immune responses.8 Aberrant functions of various immune cell types collaborate in the inflammatory processes and tissue damage in SLE,9 as summarized in Figure 3.
Figure 3. Pathophysiology of SLE*†
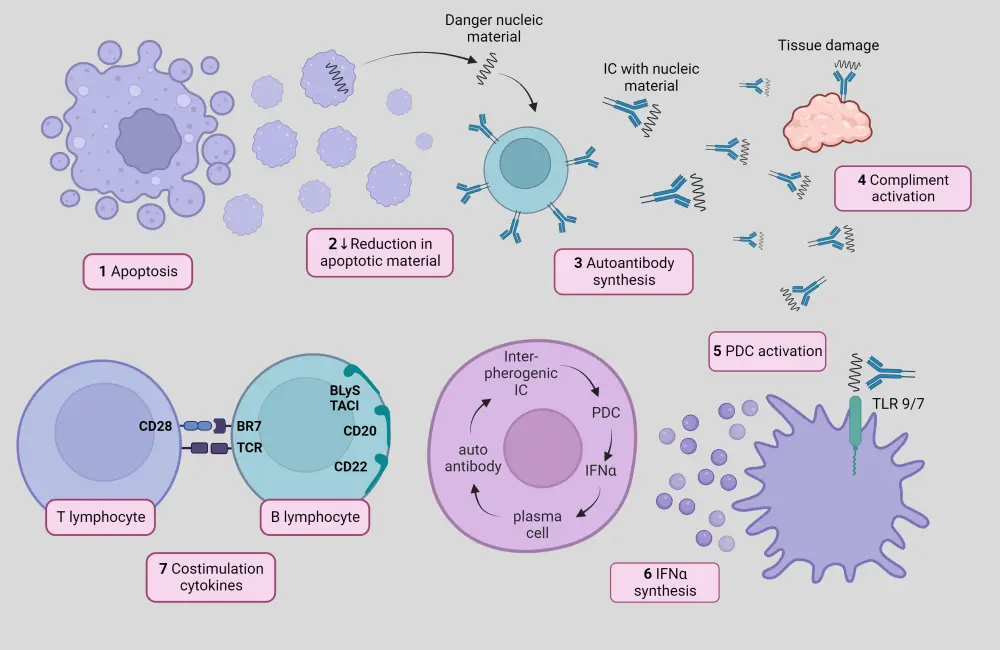
*Adapted from Sifuentes Giraldo, et al.10 Created with BioRender.com
†Altered apoptotic material clearance and NET components are recognized by autoantibodies, resulting in the formation of circulating ICs that activate the complement system and damage tissues. pDCs take up these ICs, leading to the production of type-I interferons and other pro-inflammatory cytokines. Innate immune system-triggered activation of autoreactive B and T cells perpetuates chronic inflammation and production of autoantibodies.9,10
Signs and Symptoms
SLE presents in a wide range of manifestations, varying from person to person. The most common initial presentations of SLE are illustrated in Figure 4.11 Other manifestations arising due to inflammation in different organ systems are described in our previously published article.
Figure 4. Common symptoms of SLE*
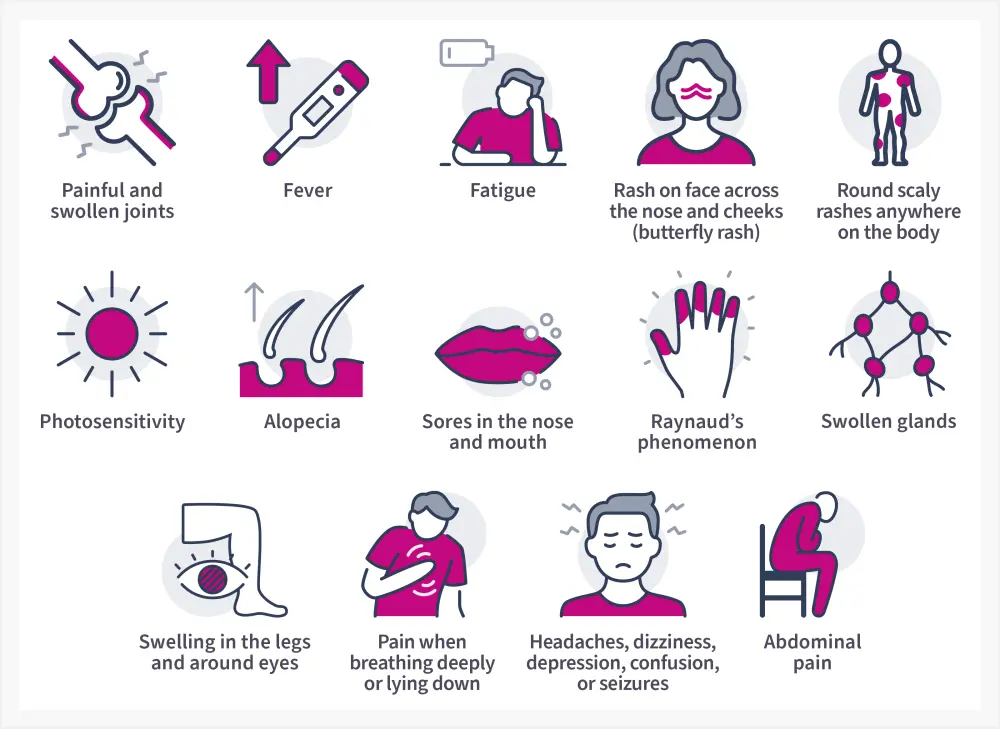
*Data from National Institute of Arthritis and Musculoskeletal and Skin Diseases.11; and CDC.5
Furthermore, SLE manifestations can be divided into two broad categories based on etiology: Type 1 and Type 2 symptoms (Figure 5).12
Figure 5. Types of SLE symptoms*
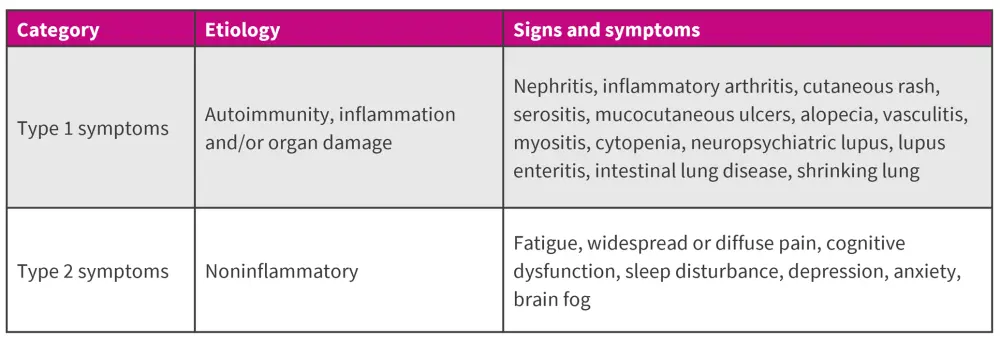
*Data from Pisetsky, et al.12
Diagnosis
The varied clinical manifestations and serological heterogeneity of SLE present diagnostic challenges,2 particularly where early signs and symptoms have nonspecific overlap with those of other diseases, potentially resulting in misdiagnosis.5 The European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) 2019 classification criteria constitute an optimized clinical approach to SLE classification that can serve as a valuable tool for clinicians in making an accurate diagnosis of SLE.2,13
Figure 6 outlines some common diagnostic techniques for SLE.
Figure 6. Diagnostic techniques for SLE*
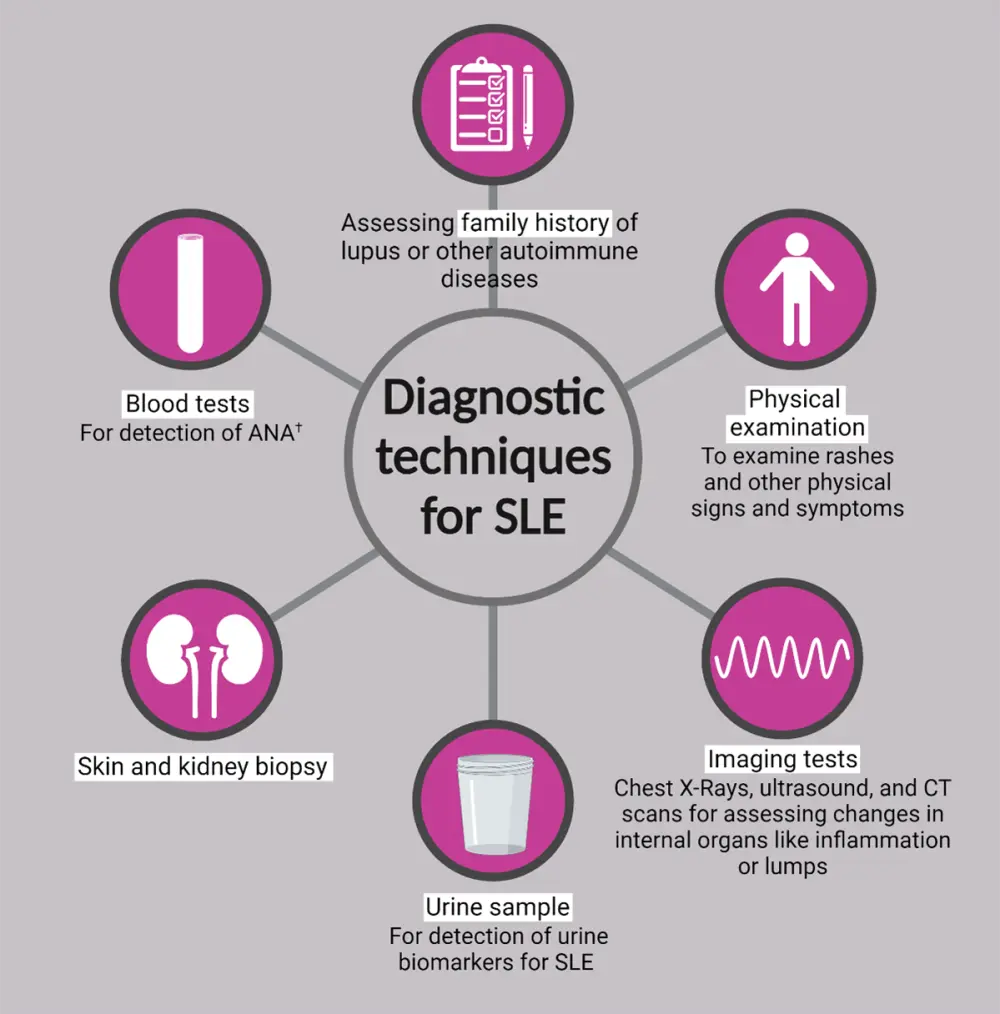
ANA, antinuclear antibody; CT, computerized tomography; ds-DNA, double-stranded deoxyribonucleic acid; Sm, Smith; SLE, systemic lupus erythematosus.
*Adapted from CDC.5; National Institute of Arthritis and Musculoskeletal and Skin Diseases.11; PR Newswire.14; Created with BioRender.com
†Indirect immunofluorescence assay, enzyme immunoassay and multiplex assay, anti-dsDNA, anti-Sm, anti-Ro (SSA), anti-La (SSB), and other serum biomarkers.
The clinical and immunological biomarkers for SLE are summarized in Figure 7.
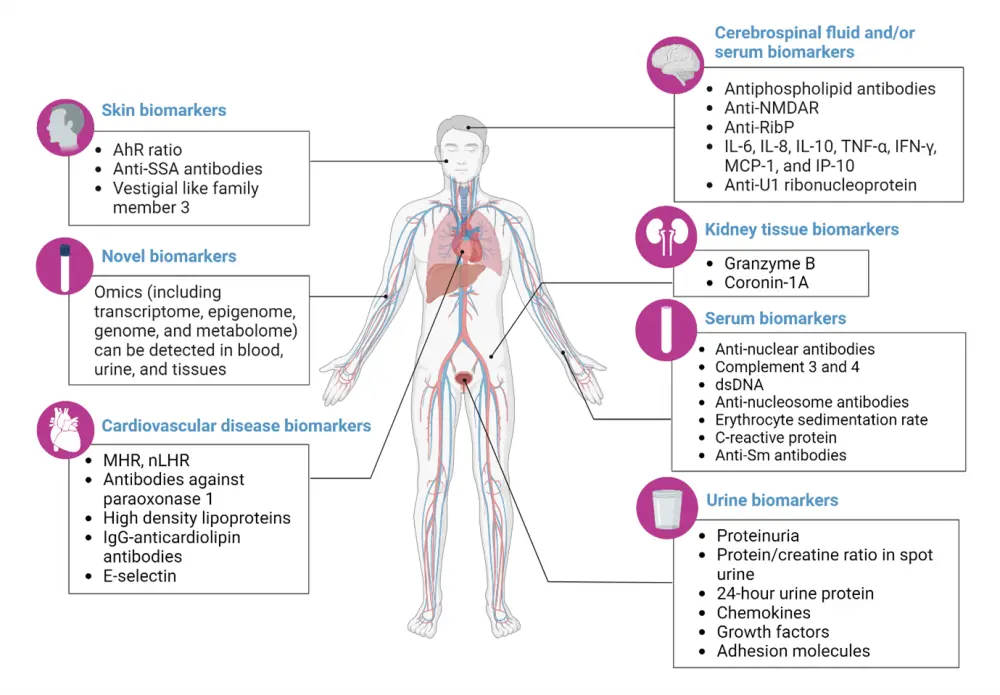
Figure 7. Clinical and immunological biomarkers for SLE*
Figure 7. Clinical and immunological biomarkers for SLE*
AhR ratio, ratio of aryl hydrocarbon receptor in Th17 cells to that in Treg; anti-NMDAR, antibodies against N-methyl-D-aspartate receptor; anti-RibP: antibodies against ribosomal proteins; anti-SSA, antibodies against Sjogren’s syndrome A; dsDNA, double-stranded DNA; IgG, immunoglobulin G; IFN, interferon; IL, interleukin; IP-10, IFN-γ-inducible protein 10; MCP-1, monocyte chemotactic pritei-1; MHR, monocyte-to-high-density lipoprotein cholesterol ratio; nLHR, low-density granulocytes-to-high-density lipoprotein cholesterol ratio; PON1, antibodies against paraoxonase1; SLE, systemic lupus erythematosus; Sm, Smith; TNF, tumor necrosis factor; Treg, regulatory T cells.
*Adapted from Yu, et al.15 Created with BioRender.com
Types of lupus
SLE is the most common form of lupus, accounting for 70% of lupus cases. Other lupus subtypes are presented in Figure 8.2,5,6
Figure 8. Types of lupus*
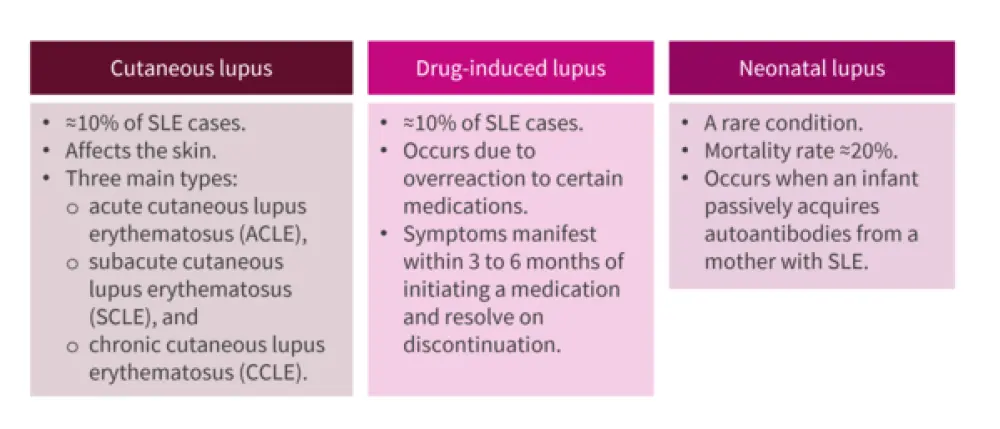
SLE, systemic lupus erythematosus.
*Data from Ameer, et al.2,5,6
One of the serious manifestations of SLE is lupus nephritis (LN), occurring in ≈40% of SLE cases.16 Based on the renal biopsy results, the LN is classified into six categories:2
Figure 9. LN classification*
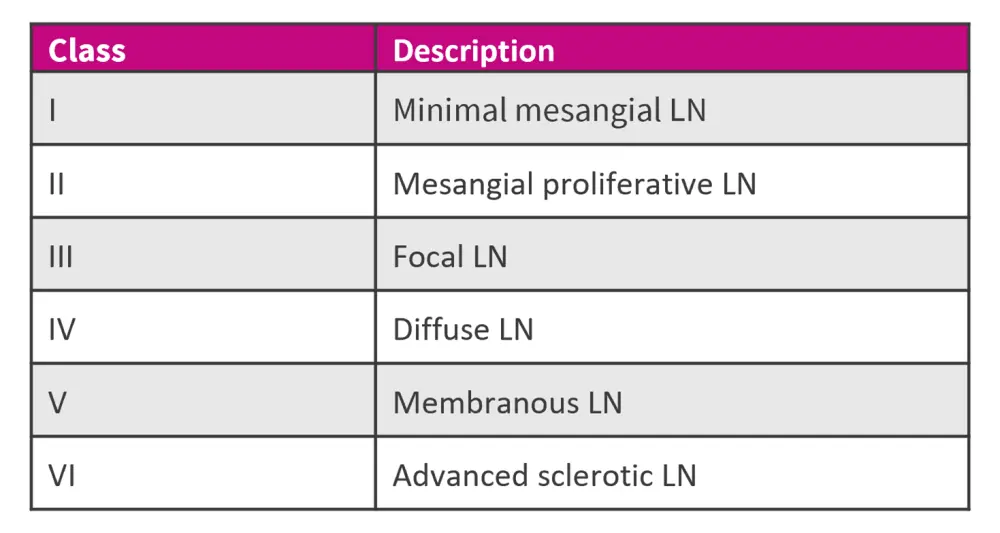
LN, lupus nephritis.
*Data from Ameer, et al.2
Management
The conventional management of SLE involves first-line treatment with antimalarials, such as hydroxychloroquine, in combination with non-steroidal anti-inflammatory drugs for mild SLE.17 In addition, treatment options involve glucocorticoids and cytotoxic or immunosuppressive agents, such as azathioprine, mycophenolate mofetil, methotrexate, cyclophosphamide, cyclosporine, and tacrolimus.9,17 Recently, voclosporin, a novel calcineurin inhibitor immunosuppressant, has been approved by the U.S. Food and Drug Administration (FDA) as an oral treatment for adults with active LN.18
The EULAR recommendations for SLE treatment have been previously published on the Lupus Hub. The management of LN as recommended by EULAR, European Renal Association (ERA), and Kidney Disease: Improving Global Outcomes (KDIGO) can be found here.
Besides standard therapy, improved understanding of the immunopathogenesis of SLE has driven the development of novel targeted immunotherapies, as outlined in Figure 10.
Figure 10. Approved and emerging targeted therapies for SLE*18,19
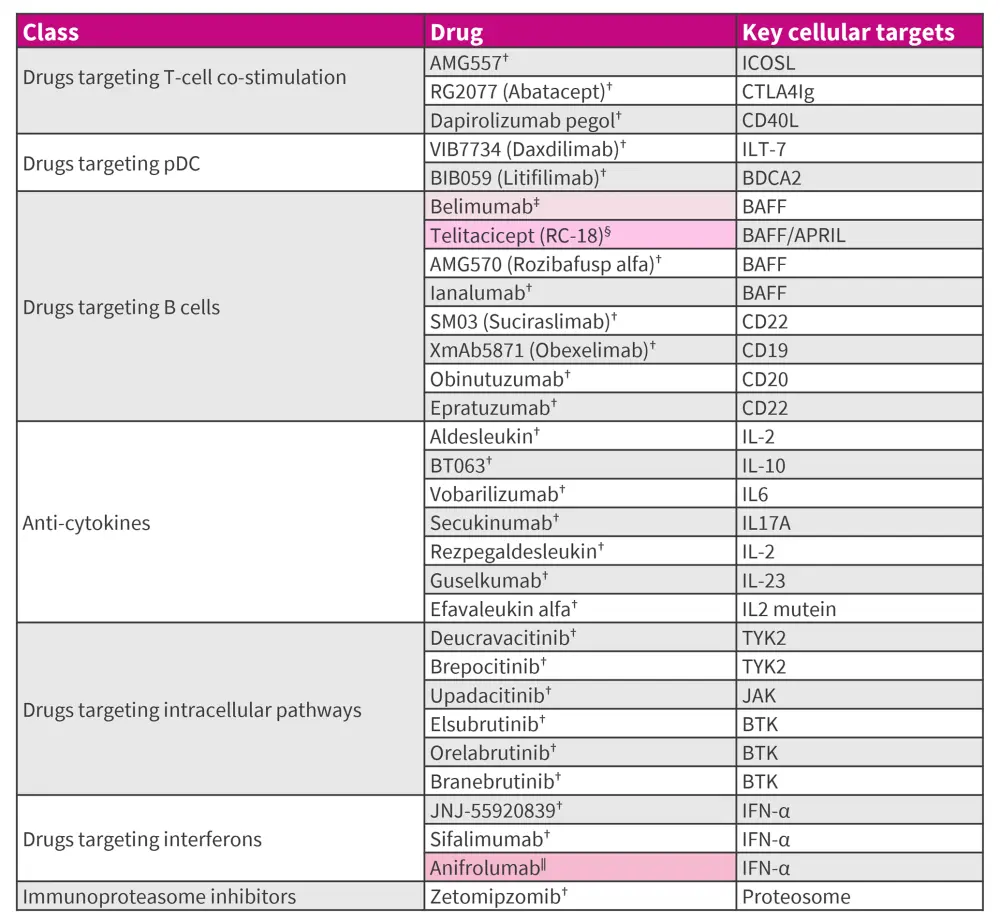
ANA, antinuclear antibody; APRIL, a proliferation-inducing ligand; BAFF, B-cell activating factor; BDCA, blood dendritic cell antigen; BTK, Bruton's tyrosine kinase; ICOSL, inducible T-cell costimulatory ligand; IFN, interferon; ILT, immunoglobulin-like transcript; JAK, Janus kinase; LN, lupus nephritis; NMPA, National Medical Products Administration; pDC, plasmacytoid dendritic cells; SLE, systemic lupus erythematosus; ST, standard therapy; TYK2, tyrosine kinase 2; US FDA, United States Food and Drug Administration.
*Data from Askanase, et al.18; Felten, et al.19; Accapezzato, et al.20; Dhillon.21; Thakare, et al.22; and Lupus.org.23
†Under clinical development.
‡US FDA approved for treating adults with ANA, active SLE, active LN and children ≥5 years with SLE and LN, in combination with ST.20
§NMPA approved in China for treatment of patients with active SLE.21
‖US FDA approved for treating adults with moderate to severe SLE, in combination with ST. Phase III clinical trial in LN undergoing.22
Furthermore, adoption of strategies such as treat-to-target (T2T) are beneficial in clinical settings to prevent damage accrual, reduce flares and mortality, as well as improve patient health-reported quality of life, as previously discussed on the Lupus Hub.
Of note, disease management and guidelines may vary across countries. Please refer to the key regional guidelines section below.
Key Guidelines and organizations
- 2019 EULAR/ACR Classification Criteria for SLE
- 2019 EULAR recommendations for management of SLE
- 2023 EULAR recommendations for management of SLE
- EULAR recommendations for non-pharmacological management of SLE
- Medical Societies/Working groups:
- Patient Organizations:
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

